Light alkanes dehydrogenation over silica supported gallium catalysts
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
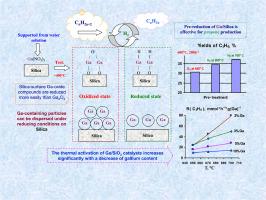
The present work is devoted to the studies of Ga/Silica catalysts in the paraffins dehydrogenation of natural gas - propane and ethane to produce valuable products - olefins and hydrogen. The gallium oxide supported on silica gel (2–10 %wt. of Ga) have been studied using XRD, TPR-H2 and STEM/EDS methods, it has been shown that the dispersion of gallium oxide over the silica surface is very effective, the support actively reacts with a gallium salt to form surface particles that are more easily reduced than pure gallium oxide. Under steady and no-stationary state the negative effect of oxidative pre-treatment and the addition of CO2 to the reaction mixture on catalytic activity was detected. On the contrary, pre-treatment of the catalysts in hydrogen (at 650–700 °C) or CO addition to the reaction mixture was accompanied by an increase in their propane dehydrogenation activities and stabilities at 600 °C. In the ethane dehydrogenation conditions (650 to 700 °C) catalysts is effective treated by hydrogen during the experiment. At 700 °C, the Ga/Silica activity may even increase during ethane dehydrogenation. By TPR-H2 it was observed that with an increase in the temperature of pre-treatment of Ga/Silica in hydrogen from 650 to 700 °C, the proportion of oxidized Ga-containing phase significantly decreases.
二氧化硅支撑镓催化剂上的轻烷脱氢反应
本研究致力于镓/二氧化硅催化剂在天然气(丙烷和乙烷)石蜡脱氢过程中的应用研究,以生产有价值的产品(烯烃和氢)。使用 XRD、TPR-H2 和 STEM/EDS 方法研究了硅胶上的氧化镓(镓含量为 2-10%wt.),结果表明氧化镓在硅胶表面的分散非常有效,支撑物会与镓盐积极反应,形成比纯氧化镓更容易还原的表面颗粒。在稳定和非稳定状态下,氧化预处理和在反应混合物中加入二氧化碳对催化活性有负面影响。相反,在氢气(650-700 °C)中对催化剂进行预处理或在反应混合物中加入 CO 的同时,催化剂的丙烷脱氢活性和在 600 °C 下的稳定性都有所提高。在乙烷脱氢条件下(650 至 700 °C),催化剂在实验过程中经过了有效的氢气处理。在 700 °C 的乙烷脱氢过程中,镓/二氧化硅的活性甚至会增加。通过 TPR-H2 可以观察到,随着镓/二氧化硅在氢气中的预处理温度从 650 ℃ 升高到 700 ℃,氧化的含镓相的比例明显降低。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


