Nd@C3N4-photoredox/chlorine dual catalyzed synthesis and evaluation of antitumor activities of 4-alkylated sulfonyl ketimines
IF 9.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The first example of Nd@C3N4-photoredox/chlorine dual catalyzed alkylation with unactivated alkanes as the alkyl sources has been developed, which allows for the synthesis of various 4-alkylated cyclic sulfonyl ketimines. In this process, chlorine functions as both a redox and hydrogen atom transfer catalyst. The synergism of the reversible Nd2+/Nd3+ and Cl¯/Cl˙ redox pairs significantly enhances overall photocatalytic efficiency. The in vitro anticancer activity of 4-alkylated products was evaluated by using the CCK8 assay against both human choroidal melanoma (MUM-2B) and lung cancer (A549) cell. Compound 3da showed approximately triple the potency of 5-fluorouracil.
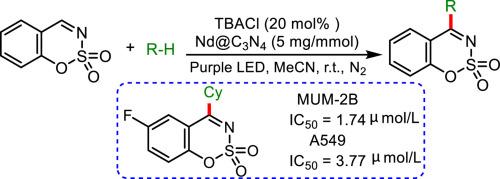
Nd@C3N4-photoredox/chlorine 双催化合成 4-烷基磺酰基酮亚胺并评估其抗肿瘤活性
首次开发出以未活化烷烃为烷基源的 Nd@C3N4-photoredox/chlorine 双催化烷基化实例,可用于合成各种 4-烷基环磺酰基酮亚胺。在这一过程中,氯既是氧化还原催化剂,又是氢原子转移催化剂。可逆的 Nd2+/Nd3+ 和 Cl¯/Cl˙ 氧化还原对的协同作用显著提高了整体光催化效率。利用 CCK8 检测法评估了 4-烷基化产物对人类脉络膜黑色素瘤(MUM-2B)和肺癌(A549)细胞的体外抗癌活性。化合物 3da 的效力约为 5-氟尿嘧啶的三倍。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


