Colorimetric fluoride ion sensors based on dipyrrolic and bipyrrolic compounds: Synthesis and anion recognition
IF 4.1
3区 工程技术
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
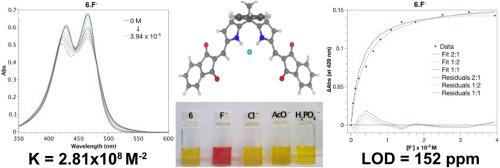
The synthesis and spectroscopic studies of four sensors for fluoride chromogenic sensing are described. The new compounds were prepared by the Knoevenagel condensation of diformyl-substituted bipyrrolic and dipyrrolic synthons ([2,2′-bipyrrole]-5,5′-dicarbaldehyde and dipyrromethane-1,9-dicarbaldehyde moieties) with malononitrile or indane-1,3-dione. They strongly absorb in the visible region and significant color changes occur in the presence of fluoride anions. Acetate and dihydrogenphosphate anions also induce observable colorimetric changes, albeit to a lesser extent. These changes, which are visible to the unaided eye, are associated with NH-bonding interactions that are unique to each anion. Non-linear regression analysis of the ground- and excited-state changes revealed anion recognition in a 2:1 stoichiometry (Host:Guest), where the electronegative character of the substituents (malononitrile or indane-1,3-dione residues) controls the sensitivity of the binding. The proposed systems all feature exceptional anion receptors that display an impressive chromogenic response through NH-bonding. Among these, compound 3 stands out with exceptionally high affinity constants of up to 7.39x10⁹ M⁻2, as well as an extremely low limit of detection at 92 ppm. NMR spectroscopy and mass spectrometry confirmed the structures of the synthesized compounds, with increased complexity in the NMR spectra due to the presence of malononitrile and indane-1,3-dione moieties. These findings highlight the potential of incorporating highly conjugated push-pull chromophores into bipyrrolic and dipyrrolic synthons for improved fluoride sensing performance in terms of both binding and signaling.
基于二吡咯和双吡咯化合物的比色氟离子传感器:合成与阴离子识别
本文介绍了四种用于氟化物色原传感的传感器的合成和光谱研究。这些新化合物是通过二甲酰基取代的双吡咯和二吡咯合成物([2,2′-双吡咯]-5,5′-二甲醛和二吡咯烷-1,9-二甲醛分子)与丙二腈或茚-1,3-二酮的克诺文纳格尔缩合反应制备的。它们在可见光区域的吸收率很高,在氟阴离子存在的情况下会发生明显的颜色变化。醋酸盐和磷酸二氢盐阴离子也会引起可观察到的比色变化,只是程度较轻。这些变化是肉眼可见的,与每种阴离子特有的 NH 键相互作用有关。对基态和激发态变化的非线性回归分析表明,阴离子识别的化学计量为 2:1(Host:Guest),其中取代基(丙二腈或茚-1,3-二酮残基)的电负性控制着结合的灵敏度。所提出的体系都具有特殊的阴离子受体,通过 NH 键结合显示出令人印象深刻的致色反应。其中,化合物 3 尤为突出,其亲和力常数高达 7.39x10𠞙 M-2,而且检测限极低,仅为 92 ppm。核磁共振光谱和质谱分析证实了合成化合物的结构,由于丙二腈和茚满-1,3-二酮分子的存在,核磁共振光谱的复杂性有所增加。这些发现凸显了在双吡咯和二吡咯合成物中加入高度共轭的推拉发色团的潜力,从而在结合和信号传导方面提高氟传感性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dyes and Pigments
工程技术-材料科学:纺织
CiteScore
8.20
自引率
13.30%
发文量
933
审稿时长
33 days
期刊介绍:
Dyes and Pigments covers the scientific and technical aspects of the chemistry and physics of dyes, pigments and their intermediates. Emphasis is placed on the properties of the colouring matters themselves rather than on their applications or the system in which they may be applied.
Thus the journal accepts research and review papers on the synthesis of dyes, pigments and intermediates, their physical or chemical properties, e.g. spectroscopic, surface, solution or solid state characteristics, the physical aspects of their preparation, e.g. precipitation, nucleation and growth, crystal formation, liquid crystalline characteristics, their photochemical, ecological or biological properties and the relationship between colour and chemical constitution. However, papers are considered which deal with the more fundamental aspects of colourant application and of the interactions of colourants with substrates or media.
The journal will interest a wide variety of workers in a range of disciplines whose work involves dyes, pigments and their intermediates, and provides a platform for investigators with common interests but diverse fields of activity such as cosmetics, reprographics, dye and pigment synthesis, medical research, polymers, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


