Synthetic studies on fusicoccin A: Enantioselective synthesis of the C-ring fragment
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
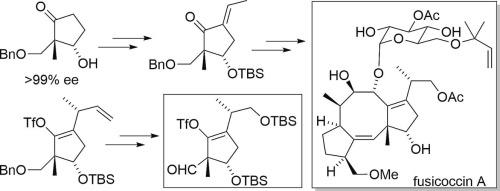
The enantioselective synthesis of the C-ring fragment of fusicoccin A is described. The TBS ether of hydroxyketone prepared by baker’s yeast reduction was converted to the α-ethylidene ketone by aldol condensation with propionaldehyde, followed by Michael reaction with divinylcopper reagent and conversion to enol triflate to afford a single diastereomer. X-ray crystallographic analysis of the crystalline derivative of the single diastereomer showed that the asymmetric carbon formed by the Michael reaction was consistent with the configuration of the C15 asymmetric carbon of fusicoccin A. The vinyl group of the enol triflate was converted to a hydroxymethyl group by selective dihydroxylation with a bulky ligand, oxidative cleavage of the resultant 1,2-diol with lead tetraacetate, and DIBAL-H reduction. The hydroxymethyl group was subsequently converted to TBS ether, followed by the hydrogenolysis of the benzyl group and Dess–Martin oxidation, accomplishing the enantioselective synthesis of the desired C-ring fragment of fusicoccin A.
夫西可辛 A 的合成研究:C 环片段的对映选择性合成
本文介绍了对映体选择性合成夫西可辛 A 的 C 环片段的方法。用面包酵母还原法制备的羟基酮的 TBS 醚通过与丙醛的醛醇缩合转化为 α-亚乙基酮,然后与二乙烯基铜试剂发生迈克尔反应并转化为三羟甲基烯醇,从而得到单一非对映异构体。对单一非对映异构体的晶体衍生物进行的 X 射线晶体分析表明,迈克尔反应形成的不对称碳与 fusicoccin A 的 C15 不对称碳的构型一致。羟甲基随后被转化为 TBS 醚,接着是苄基的氢解和 Dess-Martin 氧化,从而完成了所需的夫西可辛 A C 环片段的对映选择性合成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


