In-situ dual-exsolved nanometal anchoring on heterogeneous composite nanofiber using as SOEC cathode for direct and highly efficient CO2 electrolysis
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
An effective strategy for CO2 electrolysis by solid oxide electrolysis cells (SOECs) is to design high-performance cathode material by interface engineering. In this work, a Ni-doped Sr0.95Ti0.3Fe0.7O3-δ/Ce0.9Gd0.1O2-δ (denoted as STFN/GDCN) nanofiber composite is directly obtained via electrospinning. Then, Ni nanoparticles are dual-exsolved by 10%H2/Ar reduction to in-situ anchor on the surface of STFN and GDCN (denoted as Ni@STFN/GDCN), which is used as SOECs cathode. This developed composite cathode not only facilitates CO2 reduction reaction (CO2RR) rate but also resists thermal aggregation and carbon deposition. The Ni@STFN/GDCN cathode operating in pure CO2 with an applied voltage of 1.6 V achieves a current density of 1.85 A cm−2, surpassing most of the advanced electrodes reports by other works. Furthermore, the CO2RR testing at a current density of 1.5 A cm−2 shows no significant voltage fluctuations during 180 h, demonstrating excellent long-term stability. Our testing results show that the in-situ dual-exsolved nanometal anchoring on heterogeneous composite nanofiber is a reliable and stable SOEC cathode for direct and highly efficient CO2 electrolysis.
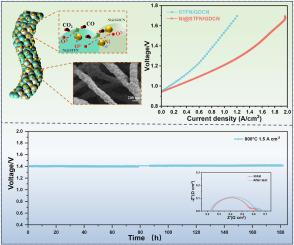
原位双溶解纳米金属锚定在异质复合纳米纤维上,作为 SOEC 阴极直接高效电解二氧化碳
利用固体氧化物电解槽(SOEC)电解二氧化碳的有效策略是通过界面工程设计高性能阴极材料。在这项工作中,通过电纺丝直接获得了掺镍的 Sr0.95Ti0.3Fe0.7O3-δ/Ce0.9Gd0.1O2-δ (简称 STFN/GDCN)纳米纤维复合材料。然后,通过 10%H2/Ar还原法将镍纳米粒子双溶解,原位锚定在STFN和GDCN表面(记为Ni@STFN/GDCN),用作SOECs阴极。所开发的这种复合阴极不仅能提高二氧化碳还原反应(CO2RR)的速率,还能防止热聚集和碳沉积。镍@STFN/GDCN阴极在纯二氧化碳中工作时,外加电压为 1.6 V,电流密度达到 1.85 A cm-2,超过了其他研究报告中的大多数先进电极。此外,在电流密度为 1.5 A cm-2 的 CO2RR 测试中,电压在 180 小时内没有明显波动,显示出出色的长期稳定性。测试结果表明,异质复合纳米纤维上的原位双溶解纳米金属锚定是一种可靠、稳定的 SOEC 阴极,可直接高效地电解二氧化碳。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


