Palladium catalyzed homocoupling reactions of gem-dibromo BODIPYs: Formation of dimer and trimer products
IF 4.1
3区 工程技术
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
We report on the synthesis and spectral properties of BODIPY 1,3-diyne dimers and 1,1-diynyl-1-alkene trimers, prepared via the Pd-catalyzed homocoupling reaction of a series of gem-dibromovinyl BODIPY (1,1-dibromo-1-alkene 4,4-difluoro-5-aryl-4-bora-3a,4a-diaza-s-indacene) dyes. The dimer and trimer moieties are connected through the ethynyl bond, attached at p-meso-phenyl or β-positions of the pyrrole ring, directly or through the phenyl spacer ring at the β-position. The assigned molecular structures of the products were confirmed using MS, 1H, 13C, 9F NMR and 11B NMR spectroscopic techniques. The absorption, fluorescence and solvatochromic properties were investigated in different solvents. The absorption maxima of unsubstituted pyrrole derivatives are bathochromic shifted as compared to the tetramethyl pyrrole substituted analogs. The highest absorption maxima were obtained when unsubstituted pyrrole 1,1-diynyl-1-alkene trimers featured a phenyl ring at the β-position. Dimers do fluoresce while trimers are void of fluorescence properties.
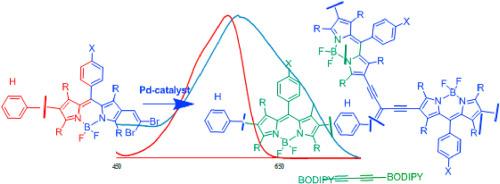
钯催化的宝石二溴 BODIPYs 同偶联反应:二聚体和三聚体产物的形成
我们报告了 BODIPY 1,3-二炔二聚体和 1,1-二炔-1-烯三聚体的合成和光谱特性。这些二聚体和三聚体是通过一系列 gem-dibromovinyl BODIPY(1,1-二溴-1-烯 4,4-二氟-5-芳基-4-bora-3a,4a-diaza-s-茚)染料的钯催化均偶联反应制备的。二聚体和三聚体通过乙炔键连接,直接连接在吡咯环的 p-间位苯基或 β 位上,或通过 β 位上的苯基间隔环连接。使用 MS、1H、13C、9F NMR 和 11B NMR 光谱技术确认了指定的产物分子结构。研究了不同溶剂中的吸收、荧光和溶色特性。与四甲基吡咯取代的类似物相比,未取代的吡咯衍生物的吸收最大值发生了浴色偏移。当未取代的吡咯-1,1-二炔基-1-烯三聚体的 β 位上有一个苯基环时,吸收最大值最高。二聚体会发出荧光,而三聚体则没有荧光特性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dyes and Pigments
工程技术-材料科学:纺织
CiteScore
8.20
自引率
13.30%
发文量
933
审稿时长
33 days
期刊介绍:
Dyes and Pigments covers the scientific and technical aspects of the chemistry and physics of dyes, pigments and their intermediates. Emphasis is placed on the properties of the colouring matters themselves rather than on their applications or the system in which they may be applied.
Thus the journal accepts research and review papers on the synthesis of dyes, pigments and intermediates, their physical or chemical properties, e.g. spectroscopic, surface, solution or solid state characteristics, the physical aspects of their preparation, e.g. precipitation, nucleation and growth, crystal formation, liquid crystalline characteristics, their photochemical, ecological or biological properties and the relationship between colour and chemical constitution. However, papers are considered which deal with the more fundamental aspects of colourant application and of the interactions of colourants with substrates or media.
The journal will interest a wide variety of workers in a range of disciplines whose work involves dyes, pigments and their intermediates, and provides a platform for investigators with common interests but diverse fields of activity such as cosmetics, reprographics, dye and pigment synthesis, medical research, polymers, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


