Infusion of fly ash in alkali salt promoted MgO-based sorbent for CO2 capture at elevated temperatures
IF 4.2
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Carbon capture at elevated temperatures (200-450°C) using MgO-based solid sorbents, typically suffers from slow kinetics and premature saturation lowering the overall uptake performance. A key factor responsible for this is the agglomeration of MgCO3 during carbonation, which could be potentially overcome by the addition of inert along with promoters. In this context, this work systematically investigates the infusion of Fly ash (FA) as inert in pure MgO and alkali-salt-promoted MgO-based sorbents prepared by the sol–gel method. The study includes characterizing the prepared sorbents based on morphological and textural properties and investigating the uptake kinetics along with cyclic performance based on thermogravimetric analysis under conditions of 250°C and 300°C for 45 mins. Among all tested modified sorbents, MgO_10NaNO3_5FA exhibited the highest uptake capacity of 14.56 mmol/g (MgO basis) followed by MgO_15NaNO3 (14.27 mmol/g) at 300°C. Cyclic studies over 10 cycles reveal higher conversion of FA-infused sorbent (MgO_10NaNO3_5FA: 59.76 %) over non-FA-infused sorbent (MgO_10NaNO3: 54.50 %) showing higher stability of the former. The results establish minimal FA infusion (5 %) in alkali nitrates promoted sorbent favorable for CO2 capture at moderate temperature while elucidating physicochemical aspects during uptake.
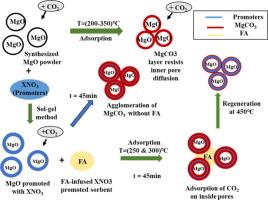
将粉煤灰注入碱盐促进的氧化镁基吸附剂,在高温下捕获二氧化碳
使用氧化镁基固体吸附剂在高温(200-450°C)条件下进行碳捕集,通常会出现动力学缓慢和过早饱和的问题,从而降低整体吸收性能。造成这种情况的一个关键因素是 MgCO3 在碳化过程中产生团聚,而通过添加惰性物质和促进剂,就有可能克服这一问题。在此背景下,本研究系统地探讨了在纯氧化镁和碱盐促进的氧化镁吸附剂中加入粉煤灰(FA)作为惰性物质的问题。研究内容包括根据形态和纹理特性对所制备的吸附剂进行表征,并在 250°C 和 300°C 条件下 45 分钟,根据热重分析法对吸附动力学和循环性能进行研究。在所有测试的改性吸附剂中,MgO_10NaNO3_5FA 在 300°C 时的吸收能力最高,达到 14.56 mmol/g(以氧化镁为基准),其次是 MgO_15NaNO3(14.27 mmol/g)。10 个周期的循环研究表明,注入 FA 的吸附剂(MgO_10NaNO3_5FA:59.76%)的转化率高于未注入 FA 的吸附剂(MgO_10NaNO3:54.50%),这表明前者具有更高的稳定性。研究结果表明,在碱硝酸盐中注入极少量的 FA(5%)可促进吸附剂在中等温度下捕获二氧化碳,同时阐明了吸附过程中的物理化学问题。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Powder Technology
工程技术-工程:化工
CiteScore
9.50
自引率
7.70%
发文量
424
审稿时长
55 days
期刊介绍:
The aim of Advanced Powder Technology is to meet the demand for an international journal that integrates all aspects of science and technology research on powder and particulate materials. The journal fulfills this purpose by publishing original research papers, rapid communications, reviews, and translated articles by prominent researchers worldwide.
The editorial work of Advanced Powder Technology, which was founded as the International Journal of the Society of Powder Technology, Japan, is now shared by distinguished board members, who operate in a unique framework designed to respond to the increasing global demand for articles on not only powder and particles, but also on various materials produced from them.
Advanced Powder Technology covers various areas, but a discussion of powder and particles is required in articles. Topics include: Production of powder and particulate materials in gases and liquids(nanoparticles, fine ceramics, pharmaceuticals, novel functional materials, etc.); Aerosol and colloidal processing; Powder and particle characterization; Dynamics and phenomena; Calculation and simulation (CFD, DEM, Monte Carlo method, population balance, etc.); Measurement and control of powder processes; Particle modification; Comminution; Powder handling and operations (storage, transport, granulation, separation, fluidization, etc.)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


