Molecular dynamics exploration of cacophony protein interactions with brood volatiles in honey bee colonies
IF 1.1
3区 农林科学
Q3 ENTOMOLOGY
引用次数: 0
Abstract
The cacophony protein is a key component of honey bee biology that has been investigated concerning hygienic behavior. The voltage-gated calcium channel subunit α1 appears to play a significant role in modulating neuronal activity associated with the detection and response to compromised brood within honey bee colonies. Despite its essential role, the molecular mechanisms underpinning hygienic behavior remain incompletely understood. Recent research suggests that the expression of the cacophony protein is linked to the efficiency of hygienic behavior, where colonies with enhanced hygienic behavior exhibit distinct patterns of cacophony expression. Understanding the role of cacophony in honey bee behavior provides insights into the molecular mechanisms underlying social interactions within bee colonies, with implications for enhancing pollination services and mitigating threats to honey bee populations. Therefore, the present study focused on unraveling and characterizing its structure and function, using bioinformatics approaches involving sequence and phylogenetic analysis. Homology modeling developed three-dimensional models of cacophony protein for Apis cerana and A. mellifera. Molecular docking studies were performed for various brood pheromones and volatiles, triggering hygienic behavior in honey bees, against cacophony protein providing computational insights into their molecular interactions. Binding free energy calculations using MM/PBSA and MM/GBSA consistently demonstrate a higher affinity of γ-octalactone for AcerCAC protein (−21.77 ± 3.01 kcal/mol and −24.81 ± 4.07 kcal/mol respectively) compared to AmelCAC protein (−20.03 ± 3.01 kcal/mol and −21.47 ± 3.19 kcal/mol respectively). This study lays a theoretical foundation for further studies regarding the mechanism of interaction of cacophony with hygienic behavior.
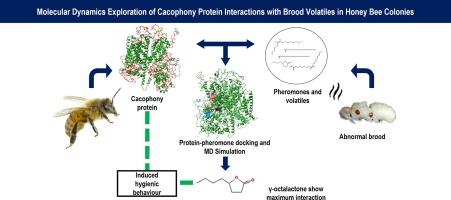
蜜蜂蜂群中蜂胶蛋白与育雏挥发物相互作用的分子动力学探索
蜂鸣蛋白是蜜蜂生物学的一个关键组成部分,已被研究用于卫生行为。电压门控钙通道亚基α1似乎在调节神经元活动方面发挥了重要作用,而这种神经元活动与蜜蜂群落中检测和应对受损的蜂巢有关。尽管α1的作用至关重要,但人们对卫生行为的分子机制仍不甚了解。最近的研究表明,嗜声蛋白的表达与卫生行为的效率有关,卫生行为增强的蜂群表现出不同的嗜声蛋白表达模式。了解 "嗡嗡声 "在蜜蜂行为中的作用,有助于深入了解蜂群内部社会互动的分子机制,对提高授粉服务和减轻对蜜蜂种群的威胁具有重要意义。因此,本研究利用生物信息学方法(包括序列和系统发育分析),重点揭示和描述其结构和功能。通过同源建模,建立了蜂王浆蛋白和蜜蜂蜂王浆蛋白的三维模型。针对引发蜜蜂卫生行为的各种育雏信息素和挥发性物质与嗜噪蛋白进行了分子对接研究,通过计算深入了解了它们的分子相互作用。利用 MM/PBSA 和 MM/GBSA 进行的结合自由能计算一致表明,与 AmelCAC 蛋白(分别为 -20.03 ± 3.01 kcal/mol 和 -21.47 ± 3.19 kcal/mol)相比,γ-辛内酯与 AcerCAC 蛋白的亲和力更高(分别为 -21.77 ± 3.01 kcal/mol 和 -24.81 ± 4.07 kcal/mol)。这项研究为进一步研究噬菌体与卫生行为的相互作用机制奠定了理论基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Asia-pacific Entomology
Agricultural and Biological Sciences-Insect Science
CiteScore
2.70
自引率
6.70%
发文量
152
审稿时长
69 days
期刊介绍:
The journal publishes original research papers, review articles and short communications in the basic and applied area concerning insects, mites or other arthropods and nematodes of economic importance in agriculture, forestry, industry, human and animal health, and natural resource and environment management, and is the official journal of the Korean Society of Applied Entomology and the Taiwan Entomological Society.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


