Metal-free carbon catalysis of toluene on activated coke and its active sources
IF 10.5
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
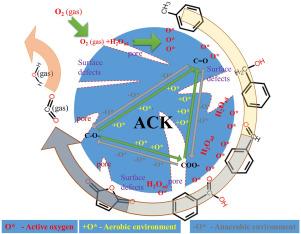
In this work, toluene removal by activated coke (ACK) was investigated. For the first time, metal-free carbon catalytic oxidation of toluene on ACK was experimentally demonstrated. On this basis, the active sources of carbon catalytic oxidation of toluene on ACK, including surface defects, pore grading degree (V/V), and surface oxygen functional groups, were identified in combination with characterization tests. The catalytic effects of different oxygen-containing functional groups (C=O, COO-, C-O-) on toluene oxidation were analyzed. The carbon catalytic oxidation reaction path of toluene on ACK has been determined as follows: toluene benzyl alcohol benzaldehyde benzoic acid maleic anhydride carbon dioxide and water. It can be divided into three steps: initial oxidation of toluene to benzyl alcohol and benzaldehyde on C=O; deep non-mineralized oxidation of benzyl alcohol and benzaldehyde on C=O, COO-, C-O-; and mineralized oxidation of intermediate products on C-O- to produce CO. The gradual oxidation of toluene promotes the interconversion of C=O, COO-, and C-O-, with the assistance of O and reactive oxygen species (O) generated by activation of O and adsorbed water.
活性焦炭上的无金属碳催化甲苯及其活性来源
本研究对活性焦(ACK)去除甲苯进行了研究。实验首次证明了无金属碳在 ACK 上催化氧化甲苯。在此基础上,结合表征测试,确定了碳在 ACK 上催化氧化甲苯的活性源,包括表面缺陷、孔隙分级度(Vmes+mac/Vt)和表面氧官能团。分析了不同含氧官能团(C=O、COO-、C-O-)对甲苯氧化的催化作用。确定了甲苯在 ACK 上的碳催化氧化反应路径如下:甲苯→苯甲醇→苯甲醛→苯甲酸→顺丁烯二酸酐→二氧化碳和水。它可分为三个步骤:甲苯在 C=O 上被初步氧化成苯甲醇和苯甲醛;苯甲醇和苯甲醛在 C=O、COO-、C-O- 上被深度非矿化氧化;中间产物在 C-O- 上被矿化氧化生成二氧化碳。甲苯的逐步氧化促进了 C=O、COO- 和 C-O- 的相互转化,O2 和活化 O2 和吸附水产生的活性氧(O∗)提供了帮助。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbon
工程技术-材料科学:综合
CiteScore
20.80
自引率
7.30%
发文量
0
审稿时长
23 days
期刊介绍:
The journal Carbon is an international multidisciplinary forum for communicating scientific advances in the field of carbon materials. It reports new findings related to the formation, structure, properties, behaviors, and technological applications of carbons. Carbons are a broad class of ordered or disordered solid phases composed primarily of elemental carbon, including but not limited to carbon black, carbon fibers and filaments, carbon nanotubes, diamond and diamond-like carbon, fullerenes, glassy carbon, graphite, graphene, graphene-oxide, porous carbons, pyrolytic carbon, and other sp2 and non-sp2 hybridized carbon systems. Carbon is the companion title to the open access journal Carbon Trends. Relevant application areas for carbon materials include biology and medicine, catalysis, electronic, optoelectronic, spintronic, high-frequency, and photonic devices, energy storage and conversion systems, environmental applications and water treatment, smart materials and systems, and structural and thermal applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


