Chemical changes from N-doped graphene and Metal-Organic Frameworks to N-G/MOF composites for improved electrocatalytic activity
IF 10.5
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Integrating N-doped graphene (N-G) with Metal-Organic Frameworks (MOFs) enhances catalytic activity for oxygen reduction reaction (ORR), often exceeding both the performances of their precursors and, in some cases, even Platinum group metal (PGM)-based catalysts. However, the factors driving this improved catalytic activity in N-G/MOF composites remain unexplored, particularly from the perspective of the chemical changes. To investigate the chemical changes in N-G and MOF upon their integration and the implications of these changes on ORR catalytic activity, an N-G/MOF was synthesized from N-G with a MOF (ZIF-8) following a mechanochemical wet ball milling process. The N-G, ZIF-8, and N-G/MOF samples were examined for changes in elemental composition, chemical state of carbon, different nitrogen and carbon bonds, and other chemical interactions. In the N-G/MOF catalyst, compared to its N-G and ZIF-8 precursors, the relative oxygen content increased, indicating the formation of additional oxygen-containing groups. The C 1s peak shifted to a lower binding energy in N-G/MOF, suggesting changes in the overall chemical or oxidation state of the carbon atoms. Besides, the increase in pyridinic-N functional groups in N-G/MOF points to the formation of additional active sites. Furthermore, the formation of C–Zn bonds in N-G/MOF suggests the probable emergence of single-atom Zn sites, while the increase in C![]() O bonds points to the formation of carboxyl or carbonyl groups. These chemical changes could be linked to the enhanced electrocatalytic activity of the N-G/MOF composite for ORR. This study may also be beneficial for other research focused on developing composite catalysts involving various N-G and MOFs-based materials.
O bonds points to the formation of carboxyl or carbonyl groups. These chemical changes could be linked to the enhanced electrocatalytic activity of the N-G/MOF composite for ORR. This study may also be beneficial for other research focused on developing composite catalysts involving various N-G and MOFs-based materials.
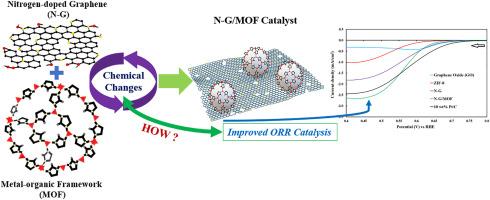
从掺杂 N 的石墨烯和金属有机框架到 N-G/MOF 复合材料的化学变化,以提高电催化活性
将掺杂 N 的石墨烯(N-G)与金属有机框架(MOFs)结合可提高氧还原反应(ORR)的催化活性,其性能往往超过其前驱体,在某些情况下甚至超过基于铂族金属(PGM)的催化剂。然而,N-G/MOF 复合材料催化活性提高的驱动因素仍有待探索,尤其是从化学变化的角度来看。为了研究 N-G 和 MOF 融合后的化学变化以及这些变化对 ORR 催化活性的影响,我们采用机械化学湿球研磨工艺,用 N-G 和 MOF(ZIF-8)合成了 N-G/MOF。研究了 N-G、ZIF-8 和 N-G/MOF 样品在元素组成、碳的化学状态、不同的氮和碳键以及其他化学相互作用方面的变化。与 N-G 和 ZIF-8 前体相比,N-G/MOF 催化剂中的相对氧含量增加,表明形成了额外的含氧基团。在 N-G/MOF 中,C 1s 峰向较低的结合能移动,表明碳原子的整体化学或氧化状态发生了变化。此外,N-G/MOF 中吡啶-N 官能团的增加表明形成了额外的活性位点。此外,N-G/MOF 中 C-Zn 键的形成表明可能出现了单原子 Zn 位点,而 CO 键的增加则表明形成了羧基或羰基。这些化学变化可能与 N-G/MOF 复合材料增强的 ORR 电催化活性有关。这项研究还可能有益于其他研究,这些研究的重点是开发涉及各种 N-G 和 MOFs 材料的复合催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbon
工程技术-材料科学:综合
CiteScore
20.80
自引率
7.30%
发文量
0
审稿时长
23 days
期刊介绍:
The journal Carbon is an international multidisciplinary forum for communicating scientific advances in the field of carbon materials. It reports new findings related to the formation, structure, properties, behaviors, and technological applications of carbons. Carbons are a broad class of ordered or disordered solid phases composed primarily of elemental carbon, including but not limited to carbon black, carbon fibers and filaments, carbon nanotubes, diamond and diamond-like carbon, fullerenes, glassy carbon, graphite, graphene, graphene-oxide, porous carbons, pyrolytic carbon, and other sp2 and non-sp2 hybridized carbon systems. Carbon is the companion title to the open access journal Carbon Trends. Relevant application areas for carbon materials include biology and medicine, catalysis, electronic, optoelectronic, spintronic, high-frequency, and photonic devices, energy storage and conversion systems, environmental applications and water treatment, smart materials and systems, and structural and thermal applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


