Sulfidation behavior of copper ferrite induced with sulfur and flotation responses
IF 4.2
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
In this study, sulfidation roasting was used to modify the copper ferrite to improve its flotation performance for the first time. The flotation results showed that the recovery of modified copper ferrite reached 91.63 %. The sulfidation mechanisms of copper ferrite at high temperatures were systematically investigated by X-ray diffraction (XRD) combining with thermodynamic calculations, X-ray photoelectron spectroscopy (XPS) and electron probe microanalysis (EPMA). XRD and thermodynamic analyses revealed that the CuFe2O4 was first reduced to compounds of Cu2O and Fe2O3, and was then converted to Cu2S and Fe3O4 with the increase of sulfur dosage at high temperatures. XPS analyses indicated that both the Cu2S and CuSO4 were mainly formed on the surface of mineral after the sulfidation treatment. The EPMA analyses confirmed that Cu2S was generated at the outer layer of samples after the sulfidation, while the inner part of particles was mainly composed of Fe3O4. It is concluded that the sulfidation reaction of CuFe2O4 occurred from the surface to interior during the thermal process. The sulfidation reaction pathway was devised as the follows: CuO·Fe2O3 → CuOx·Fe2O3(0 < x < 1) → Cu2O + Fe2O3 → Cu2S + Fe2O3 → Cu2S + Fe3O4 to better interpret the transformation mechanisms of CuFe2O4. These results will provide a good theoretical basis for the recovery of Cu and Fe from the refractory oxide copper resource by the combined methods of sulfidation and flotation.
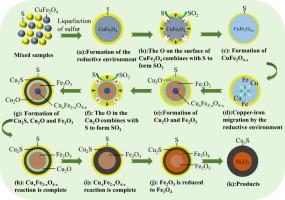
硫诱导铜铁氧体的硫化行为和浮选反应
本研究首次采用硫化焙烧对铜铁氧体进行改性,以改善其浮选性能。浮选结果表明,改性铁氧体铜的回收率达到 91.63%。通过 X 射线衍射(XRD)结合热力学计算、X 射线光电子能谱(XPS)和电子探针显微分析(EPMA)系统地研究了铜铁氧体在高温下的硫化机理。X 射线衍射和热力学分析表明,CuFe2O4 首先被还原成 Cu2O 和 Fe2O3 的化合物,然后在高温下随着硫剂量的增加转化成 Cu2S 和 Fe3O4。XPS 分析表明,硫化处理后,Cu2S 和 CuSO4 主要在矿物表面形成。EPMA 分析证实,硫化后 Cu2S 在样品外层生成,而颗粒内部主要由 Fe3O4 组成。由此得出结论,在热处理过程中,CuFe2O4 的硫化反应是由表及里的。硫化反应路径设计如下:CuO-Fe2O3 → CuOx-Fe2O3(0 < x < 1) → Cu2O + Fe2O3 → Cu2S + Fe2O3 → Cu2S + Fe3O4,从而更好地解释了 CuFe2O4 的转化机理。这些结果将为采用硫化和浮选相结合的方法从难选氧化铜资源中回收铜和铁提供良好的理论基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Powder Technology
工程技术-工程:化工
CiteScore
9.50
自引率
7.70%
发文量
424
审稿时长
55 days
期刊介绍:
The aim of Advanced Powder Technology is to meet the demand for an international journal that integrates all aspects of science and technology research on powder and particulate materials. The journal fulfills this purpose by publishing original research papers, rapid communications, reviews, and translated articles by prominent researchers worldwide.
The editorial work of Advanced Powder Technology, which was founded as the International Journal of the Society of Powder Technology, Japan, is now shared by distinguished board members, who operate in a unique framework designed to respond to the increasing global demand for articles on not only powder and particles, but also on various materials produced from them.
Advanced Powder Technology covers various areas, but a discussion of powder and particles is required in articles. Topics include: Production of powder and particulate materials in gases and liquids(nanoparticles, fine ceramics, pharmaceuticals, novel functional materials, etc.); Aerosol and colloidal processing; Powder and particle characterization; Dynamics and phenomena; Calculation and simulation (CFD, DEM, Monte Carlo method, population balance, etc.); Measurement and control of powder processes; Particle modification; Comminution; Powder handling and operations (storage, transport, granulation, separation, fluidization, etc.)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


