Tailoring hydrogen adsorption via charge transfer at bimetallic Cr0.48Ru0.52 alloy nanoparticles decorated on carbon nanofiber for enhanced hydrogen evolution catalysis
IF 10.5
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Designing and synthesizing highly efficient and stable electrocatalysts for the hydrogen evolution reaction (HER) is crucial for the practical and large-scale application of hydrogen sources. Recent research has focused on tuning the electronic structure of electrocatalysts to achieve optimal HER activity, with particular emphasis on interfacial engineering to induce electron transfer and optimize HER kinetics. In this study, as part of research into heterointerface engineering, bimetallic Cr0.48Ru0.52 alloy nanoparticles decorated on carbon nanofibers (Cr0.48Ru0.52/CNFs) were fabricated through a simple electrospinning and post-calcination process to serve as an efficient alkaline HER catalyst. The Cr0.48Ru0.52/CNFs demonstrated exceptional electrocatalytic HER performance, with an overpotential of only 13 mV at −10 mA cm−2 and a Tafel slope of 60.8 mV dec−1, indicating high catalytic activity compared to commercial benchmark catalysts (i.e., Ru/C and Pt/C). First-principles density functional theory calculations support these results, revealing that Cr0.48Ru0.52 balances proton reduction (Volmer step) and H∗ desorption (Tafel/Heyrovsky step) processes during electrocatalysis, as evidenced by the near-zero hydrogen adsorption (ΔGH∗) value (ca. −0.11 eV). Therefore, this study highlights that Cr0.48Ru0.52/CNFs, with noble Ru comprising only half of the total metal content, can promote optimal HER kinetics under alkaline condition.
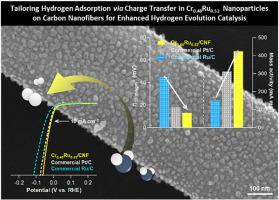
通过碳纳米纤维上装饰的双金属 Cr0.48Ru0.52 合金纳米粒子上的电荷转移定制氢吸附,以增强氢气进化催化作用
设计和合成高效稳定的氢进化反应(HER)电催化剂对于氢源的实际和大规模应用至关重要。近期研究的重点是调整电催化剂的电子结构,以实现最佳的氢进化反应活性,尤其强调通过界面工程来诱导电子转移和优化氢进化反应动力学。在本研究中,作为异质界面工程研究的一部分,通过简单的电纺丝和后煅烧工艺,制备了装饰在碳纳米纤维上的双金属 Cr0.48Ru0.52 合金纳米粒子(Cr0.48Ru0.52/CNFs),作为一种高效的碱性 HER 催化剂。Cr0.48Ru0.52/CNFs 具有优异的电催化 HER 性能,在 -10 mA cm-2 条件下过电位仅为 13 mV,Tafel 斜率为 60.8 mV dec-1,与商业基准催化剂(即 Ru/C 和 Pt/C)相比具有很高的催化活性。第一原理密度泛函理论计算支持这些结果,揭示了 Cr0.48Ru0.52 在电催化过程中平衡了质子还原(Volmer 步骤)和 H∗ 解吸(Tafel/Heyrovsky 步骤)过程,氢吸附 (ΔGH∗) 值(约 -0.11 eV)接近零就是证明。因此,本研究强调,Cr0.48Ru0.52/CNFs(惰性 Ru 仅占金属总含量的一半)可在碱性条件下促进最佳的 HER 动力学。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbon
工程技术-材料科学:综合
CiteScore
20.80
自引率
7.30%
发文量
0
审稿时长
23 days
期刊介绍:
The journal Carbon is an international multidisciplinary forum for communicating scientific advances in the field of carbon materials. It reports new findings related to the formation, structure, properties, behaviors, and technological applications of carbons. Carbons are a broad class of ordered or disordered solid phases composed primarily of elemental carbon, including but not limited to carbon black, carbon fibers and filaments, carbon nanotubes, diamond and diamond-like carbon, fullerenes, glassy carbon, graphite, graphene, graphene-oxide, porous carbons, pyrolytic carbon, and other sp2 and non-sp2 hybridized carbon systems. Carbon is the companion title to the open access journal Carbon Trends. Relevant application areas for carbon materials include biology and medicine, catalysis, electronic, optoelectronic, spintronic, high-frequency, and photonic devices, energy storage and conversion systems, environmental applications and water treatment, smart materials and systems, and structural and thermal applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


