Unveiling the effect of cycle time and ambient temperature on the failure mechanisms of commercial LiCoO2/artificial graphite pouch cells
IF 8.6
2区 工程技术
Q1 ENERGY & FUELS
引用次数: 0
Abstract
Many efforts have been devoted to investigate failure mechanisms of LiCoO2-based lithium-ion batteries at ≥4.55 vs. Li+/Li. However, most of these works are conducted on coin-type half-cells and rarely consider the effects of cycle time and ambient temperature on mechanisms. So, it calls an alarming demand for making clear the cyclabilities of cells at different temperatures, then differentiating the root causes for their capacity degradations. Herein, the cyclabilities of an ∼3.1 Ah commercial LiCoO2/artificial graphite pouch cell at 3–4.45 V are compared at 25 and 60 °C. It is found that the cycle lives at 25 and 60 °C are 2944 and 122 cycles, respectively. The structural variations of the cycled electrodes are systematically studied. Results reveal that the main cause for the capacity beginning to decline after 3000 cycles at 25 °C is the damage of LiCoO2 structure, while its impedance growth and other side reactions are not severe. Differently, the capacity dropping upon cycling at 60 °C is caused by the parasitic reactions including successive electrolyte decomposition, severe cobalt dissolution and Li deposition. Obviously, these findings can provide a theoretical basis for further optimizing of LiCoO2 cells.
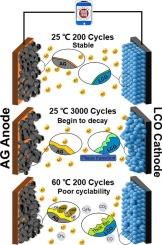
揭示循环时间和环境温度对商用钴酸锂/人造石墨袋电池失效机制的影响
许多人致力于研究钴酸锂基锂离子电池在≥4.55vs.Li+/Li条件下的失效机理。然而,这些研究大多针对纽扣型半电池,很少考虑循环时间和环境温度对失效机理的影响。因此,迫切需要明确电池在不同温度下的循环能力,进而区分电池容量衰减的根本原因。在此,比较了 3.1 Ah 的商用钴酸锂/人造石墨袋电池在 3-4.45 V 和 25 和 60 °C 下的循环能力。结果发现,在 25 ℃ 和 60 ℃ 下的循环寿命分别为 2944 次和 122 次。对循环电极的结构变化进行了系统研究。结果表明,在 25 ℃ 下循环 3000 次后容量开始下降的主要原因是钴酸锂结构的损坏,而其阻抗增长和其他副反应并不严重。不同的是,在 60 °C 下循环后容量下降是由寄生反应引起的,包括连续电解质分解、严重的钴溶解和锂沉积。显然,这些发现为进一步优化钴酸锂电池提供了理论依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Sustainable Materials and Technologies
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
13.40
自引率
4.20%
发文量
158
审稿时长
45 days
期刊介绍:
Sustainable Materials and Technologies (SM&T), an international, cross-disciplinary, fully open access journal published by Elsevier, focuses on original full-length research articles and reviews. It covers applied or fundamental science of nano-, micro-, meso-, and macro-scale aspects of materials and technologies for sustainable development. SM&T gives special attention to contributions that bridge the knowledge gap between materials and system designs.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


