Dual storage mechanism of Bi2O3/Co3O4/MWCNT composite as an anode for lithium-ion battery and lithium-ion capacitor
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Bismuth oxide(Bi2O3) and cobalt oxide(Co3O4) are promising owing to their unique properties, high storage capacity, low cost, and eco-friendliness, making them ideal for lithium-ion batteries(LIBs) and lithium-ion capacitors(LICs) anodes. This study presents the synthesis and thorough characterization of Bi2O3/Co3O4 and Bi2O3/Co3O4/MWCNT composites as potential LIB and LIC anode materials. The materials are synthesized using a hydrothermal process succeeded by annealing. Structural, morphological, and compositional studies were analyzed. Various tests evaluated electrochemical performance, including cyclic voltammetry(CV), confirming a dual storage mechanism like alloying and conversion reaction involved for better energy storage. Specific discharge capacities of 834 mAh/g and 1184 mAh/g were recorded for Bi2O3/Co3O4 and Bi2O3/Co3O4/MWCNT composite electrodes at a current density of 100 mA/g, respectively. The composite material exhibited notably enhanced rate capability, with 31 % and 51 % discharge capacities for Bi2O3/Co3O4 and Bi2O3/Co3O4/MWCNT, respectively. The cyclic stability assessment revealed that Bi2O3/Co3O4 and Bi2O3/Co3O4/MWCNT maintained a high coulombic efficiency of around 99 % over 250 charge–discharge cycles at a high current density of 1 A/g. The capacity retention was approximately 253 mAh/g for Bi2O3/Co3O4 and 439 mAh/g for the Bi2O3/Co3O4/MWCNT composite, indicating excellent cyclic stability and minimal energy loss during cycling. Moreover, the LICs assembly of Bi2O3/Co3O4/MWCNT//CB was investigated, revealing a power density of 200 W kg−1 alongside an energy density of 8.64 Wh kg−1. The cyclic stability assessment over 10,000 cycles exhibits a capacity retention of approximately 45 % under a high current density of 2 A/g.
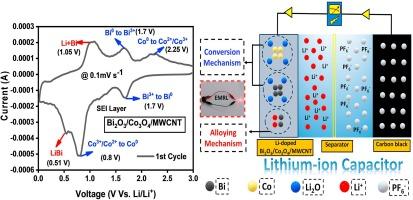
作为锂离子电池和锂离子电容器负极的 Bi2O3/Co3O4/MWCNT 复合材料的双重存储机制
氧化铋(Bi2O3)和氧化钴(Co3O4)因其独特的性质、高存储容量、低成本和生态友好性而大有可为,是锂离子电池(LIB)和锂离子电容器(LIC)阳极的理想材料。本研究介绍了 Bi2O3/Co3O4 和 Bi2O3/Co3O4/MWCNT 复合材料的合成和全面表征。这些材料采用水热法合成,然后进行退火处理。分析了结构、形态和成分研究。各种测试评估了电化学性能,包括循环伏安法(CV),证实了合金化和转换反应等双重储能机制,从而实现了更好的储能。在电流密度为 100 mA/g 时,Bi2O3/Co3O4 和 Bi2O3/Co3O4/MWCNT 复合电极的比放电容量分别为 834 mAh/g 和 1184 mAh/g。复合材料的速率能力明显增强,Bi2O3/Co3O4 和 Bi2O3/Co3O4/MWCNT 的放电容量分别为 31% 和 51%。循环稳定性评估显示,在 1 A/g 的高电流密度下,Bi2O3/Co3O4 和 Bi2O3/Co3O4/MWCNT 在 250 次充放电循环中保持了约 99% 的高库仑效率。Bi2O3/Co3O4 的容量保持率约为 253 mAh/g,Bi2O3/Co3O4/MWCNT 复合材料的容量保持率约为 439 mAh/g,这表明其具有出色的循环稳定性,循环过程中的能量损失极小。此外,还对 Bi2O3/Co3O4/MWCNT//CB 的 LICs 组件进行了研究,结果显示功率密度为 200 W kg-1,能量密度为 8.64 Wh kg-1。循环稳定性评估显示,在 2 A/g 的高电流密度下,10000 次循环的容量保持率约为 45%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


