Surface-selenization formed NiFe MOF@NiSex heterogeneous arrays for enhanced oxygen evolution and methanol electrooxidation
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Designing reasonable electrocatalysts for oxygen evolution reaction (OER) is a vital issue for water splitting to hydrogen. We report a surface-selenization of NiFe MOF-74 formed NiFe MOF@NiSex arrays through the simple solvothermal method. The NiFe MOF@NiSex heterostructures greatly enhance the charge transfer and cooperativity of active sites and result in strong adsorption capacity for OH– to strongly boost the OER and MOR process. The optimized electrode shows the highly efficient catalytic activity for OER with a low onset potential of 1.31 V vs. RHE and small Tafel slopes of 38.3 mV dec−1 in alkaline media. And it shows the extremely low overpotential of 229 and 329 mV at 100 and 500 mA cm−2. Moreover, its current density can reach more than 500 mA cm−2 at the potentials of 1.645 V vs. RHE at 0.8 M methanol/1 M KOH electrolyte, and it shows very good long-term stability at large current density. This heterogeneous arrays electrocatalysts may play a positive role in energy conversion and storage process.
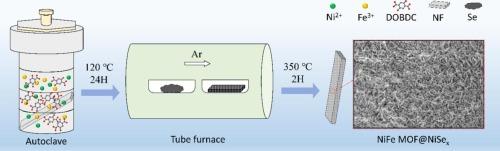
用于增强氧进化和甲醇电氧化的表面细化形成的 NiFe MOF@NiSex 异构阵列
设计合理的氧进化反应(OER)电催化剂是水分离制氢的关键问题。我们报告了通过简单的溶解热法对 NiFe MOF-74 进行表面硒化形成 NiFe MOF@NiSex 阵列的过程。NiFe MOF@NiSex 异质结构极大地增强了活性位点的电荷转移和协同性,从而产生了对 OH- 的强大吸附能力,有力地促进了 OER 和 MOR 过程。优化后的电极在碱性介质中具有 1.31 V 对 RHE 的低起始电位和 38.3 mV dec-1 的小 Tafel 斜坡,显示出高效的 OER 催化活性。在 100 mA cm-2 和 500 mA cm-2 条件下,过电位分别为 229 mV 和 329 mV。此外,在 0.8 M 甲醇/1 M KOH 电解液中,其对 RHE 的电位为 1.645 V 时,电流密度可达到 500 mA cm-2 以上,并且在大电流密度下表现出很好的长期稳定性。这种异质阵列电催化剂可在能量转换和储存过程中发挥积极作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


