The strand exchange domain of tumor suppressor PALB2 is intrinsically disordered and promotes oligomerization-dependent DNA compaction
IF 4.6
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
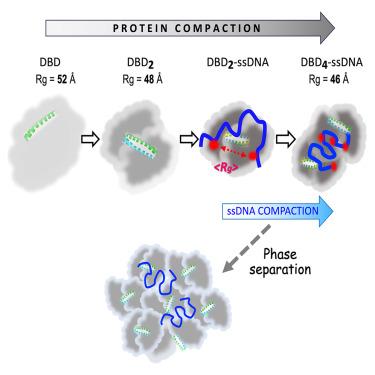
The partner and localizer of BRCA2 (PALB2) is a scaffold protein linking BRCA1 with BRCA2 and RAD51 during homologous recombination (HR). PALB2 interaction with DNA strongly enhances HR in cells, while the PALB2 DNA-binding domain (PALB2-DBD) supports DNA strand exchange in vitro. We determined that PALB2-DBD is intrinsically disordered beyond a single N-terminal α-helix. Coiled-coil mediated dimerization is stabilized by interaction between intrinsically disordered regions (IDRs) leading to a 2-fold structural compaction. Single-stranded (ss)DNA binding promotes additional structural compaction and protein tetramerization. Using confocal single-molecule FRET, we observed bimodal and oligomerization-dependent compaction of ssDNA bound to PALB2-DBD, suggesting a novel strand exchange mechanism. Bioinformatics analysis and preliminary observations indicate that PALB2 forms protein-nucleic acids condensates. Intrinsically disordered DBDs are prevalent in the human proteome. PALB2-DBD and similar IDRs may use a chaperone-like mechanism to aid formation and resolution of DNA and RNA multichain intermediates during DNA replication, repair and recombination.
肿瘤抑制因子 PALB2 的链交换结构域具有内在无序性,可促进寡聚化依赖性 DNA 压实
BRCA2 的伙伴和定位器(PALB2)是同源重组(HR)过程中连接 BRCA1 与 BRCA2 和 RAD51 的支架蛋白。PALB2 与 DNA 的相互作用可强烈增强细胞中的 HR,而 PALB2 DNA 结合域(PALB2-DBD)则支持体外 DNA 链交换。我们确定,PALB2-DBD 除了单个 N 端 α 螺旋外,在本质上是无序的。盘绕线圈介导的二聚化通过内在无序区(IDRs)之间的相互作用而稳定,从而导致结构压缩 2 倍。单链(ss)DNA 的结合促进了额外的结构压实和蛋白质四聚化。利用共聚焦单分子 FRET,我们观察到了与 PALB2-DBD 结合的 ssDNA 的双峰和寡聚依赖性压实,这表明了一种新的链交换机制。生物信息学分析和初步观察表明,PALB2 形成了蛋白质-核酸凝聚体。本质上无序的 DBD 在人类蛋白质组中非常普遍。PALB2-DBD和类似的IDRs可能利用类似于伴侣的机制,在DNA复制、修复和重组过程中帮助DNA和RNA多链中间体的形成和解析。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


