Adsorption and work function type sensing of SF6 decompositions (SO2, SOF2, SO2F2, H2S and HF) based on Fe and Cu decorated B4CN3 monolayer. A first-principles study
IF 2
3区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
During frequent arc-quenching operations, SF6 gas in SF6 power circuit breakers decomposes into sulfur-based gases, which eventually causes weakening of quenching ability of SF6 gas. Therefore, such decompositions must be sensed and captured timely to avoid any malfunctioning of SF6 circuit breaker. Here we investigate the sensing/adsorption and release of SF6 decompositions (SO2, SOF2, SO2F2, H2S, and HF) on a monolayer B4CN3 through first-principles calculations. Pure B4CN3 is unable to capture gas molecules due to insufficient adsorption energy, resulting in their rapid release. However, with Fe and Cu decoration on B4CN3, adsorption and release are greatly improved. The mechanism included evaluating band structure, work functions (Φ), and transport transmission (I-V). The order of gas adsorption/sensitivity of Fe and Cu decorated B4CN3 to target gases is SOF2 > SO2F2 > SO2 > H2S > HF. Moreover, despite having lower adsorption energies, Cu decorated B4CN3 is more sensitive to all gas molecules than Fe decorated, indicating no direct impact of higher adsorption energy to sensitivity. Gas molecule release time at room temperature can be reduced to nanoseconds either by rising the temperature to 499 K or by just exposing to UV radiation. Our study presents theoretical insights into the gas sensing capabilities of Fe and Cu decorated B4CN3 monolayers for SF6 decompositions.
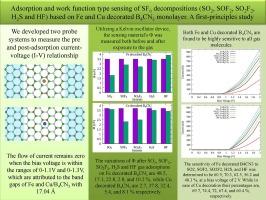
基于铁和铜装饰的 B4CN3 单层对 SF6 分解(SO2、SOF2、SO2F2、H2S 和 HF)的吸附和功函数式传感。第一原理研究
在频繁的熄弧操作中,SF6 电力断路器中的 SF6 气体会分解成硫基气体,最终导致 SF6 气体的熄弧能力减弱。因此,必须及时感应和捕捉这种分解,以避免 SF6 断路器出现任何故障。在此,我们通过第一原理计算研究了单层 B4CN3 对 SF6 分解物(SO2、SOF2、SO2F2、H2S 和 HF)的感应/吸附和释放。纯 B4CN3 由于吸附能量不足而无法捕获气体分子,导致气体分子迅速释放。然而,在 B4CN3 上装饰铁和铜后,吸附性和释放性大大提高。研究机制包括评估带状结构、功函数(Φ)和传输传输(I-V)。铁和铜装饰的 B4CN3 对目标气体的吸附/敏感性顺序为 SOF2 > SO2F2 > SO2 > H2S > HF。此外,尽管铜装饰的 B4CN3 的吸附能较低,但它对所有气体分子的灵敏度都高于铁装饰的 B4CN3,这表明吸附能越高对灵敏度没有直接影响。通过将温度升高到 499 K 或直接暴露在紫外线辐射下,室温下的气体分子释放时间可缩短到纳秒级。我们的研究从理论上揭示了铁和铜装饰的 B4CN3 单层对 SF6 分解的气体传感能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
4.60
自引率
4.30%
发文量
278
审稿时长
39 days
期刊介绍:
Chemical Physics publishes experimental and theoretical papers on all aspects of chemical physics. In this journal, experiments are related to theory, and in turn theoretical papers are related to present or future experiments. Subjects covered include: spectroscopy and molecular structure, interacting systems, relaxation phenomena, biological systems, materials, fundamental problems in molecular reactivity, molecular quantum theory and statistical mechanics. Computational chemistry studies of routine character are not appropriate for this journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


