Distinct effects of sacituzumab govitecan and berzosertib on DNA damage response in ovarian cancer
IF 4.6
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Antibody–drug conjugates (ADCs) have become an important class of anticancer drugs in solid tumors including drug-resistant gynecologic malignancies. TROP2 is a cell surface antigen that is highly expressed in ovarian carcinoma (OC) but minimally expressed in normal ovarian tissues. In this study, we aimed to identify how TROP2-specific ADC, sacituzumab govitecan (SG), modulates DNA damage response pathways in drug-resistant OC. We found that SG induces G2/M arrest, increases RPA1 foci, and decreases replication fork speed, resulting in replication stress in TROP2-positive cells while these were less evident in TROP2-negative cells. In OC in vitro and in vivo models, SN-38 sensitivity and TROP2 expression play key roles in response to either ATR inhibitor or SG alone, or in combination. Additionally, inhibition of translesion DNA synthesis enhances SG and PARP inhibitor (PARPi) sensitivity in PARPi-resistant OC cells. These findings provide mechanistic insights for clinical development of SG in drug-resistant OC.
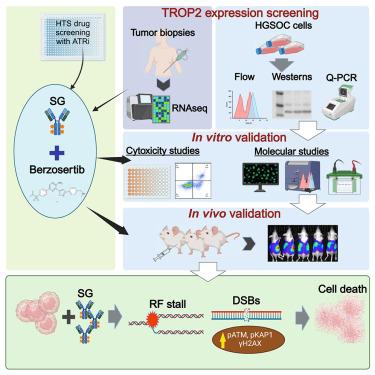
萨希珠单抗-戈维替康和贝瑞沙替布对卵巢癌 DNA 损伤反应的不同影响
抗体药物结合物(ADC)已成为治疗实体瘤(包括耐药妇科恶性肿瘤)的一类重要抗癌药物。TROP2 是一种细胞表面抗原,在卵巢癌(OC)中高表达,但在正常卵巢组织中表达极少。在这项研究中,我们旨在确定 TROP2 特异性 ADC--sacituzumab govitecan(SG)如何调节耐药 OC 的 DNA 损伤反应通路。我们发现,在TROP2阳性细胞中,SG诱导G2/M停滞,增加RPA1病灶,降低复制叉速度,从而导致复制压力,而在TROP2阴性细胞中这些作用并不明显。在 OC 体外和体内模型中,SN-38 的敏感性和 TROP2 的表达对 ATR 抑制剂或 SG 的单独或联合反应起着关键作用。此外,在 PARPi 抗性 OC 细胞中,抑制转子 DNA 合成可增强 SG 和 PARP 抑制剂(PARPi)的敏感性。这些发现为SG在耐药OC中的临床开发提供了机理上的启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


