Reprogramming of fibroblasts into cancer-associated fibroblasts via IGF2-mediated autophagy promotes metastasis of lung cancer cells
IF 4.6
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Cancer-associated fibroblasts (CAFs) are major component of stromal cells. Growing evidence suggests that CAFs promote tumor growth and metastasis; however, the reprogramming of normal fibroblasts (NFs) into CAFs by tumor cells still remains largely unknown. In this study, we found that non-small cell lung cancer (NSCLC) cells activated NFs into CAFs via autophagy induction. Insulin-like growth factor 2 (IGF2) secreted by NSCLC cells mediated NSCLC cells’ effect on autophagy induction and CAFs activation. Importantly, the activated CAFs promoted NSCLC cells growth, migration, and invasion. Further study showed that the activated CAFs facilitated NSCLC cells invasion via promoting epithelial-mesenchymal transition (EMT) process, upregulating metastasis-related genes, releasing CXCL12, and activating its downstream AKT serine/threonine kinase 1 (AKT)/ nuclear factor κB (NF-κB) signaling pathway. These findings revealed that IGF2-mediated autophagy plays a critical role in CAFs activation and suggested the IGF2-autophagy cascade in fibroblasts could be a potential target for lung cancer therapy.
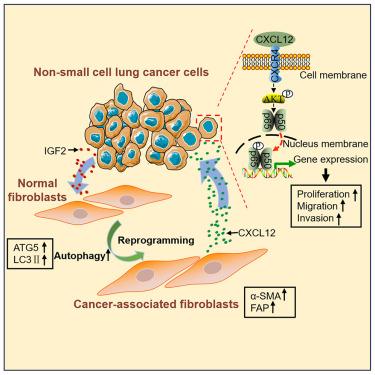
通过 IGF2 介导的自噬作用将成纤维细胞重编程为癌症相关成纤维细胞,促进肺癌细胞的转移
癌症相关成纤维细胞(CAFs)是基质细胞的主要组成部分。越来越多的证据表明,CAFs 可促进肿瘤生长和转移;然而,肿瘤细胞将正常成纤维细胞(NFs)重编程为 CAFs 的过程在很大程度上仍不为人知。在这项研究中,我们发现非小细胞肺癌(NSCLC)细胞通过自噬诱导将 NFs 活化为 CAFs。NSCLC细胞分泌的胰岛素样生长因子2(IGF2)介导了NSCLC细胞对自噬诱导和CAFs活化的影响。重要的是,活化的CAFs促进了NSCLC细胞的生长、迁移和侵袭。进一步的研究表明,活化的CAFs通过促进上皮-间质转化(EMT)过程、上调转移相关基因、释放CXCL12以及激活其下游的AKT丝氨酸/苏氨酸激酶1(AKT)/核因子κB(NF-κB)信号通路来促进NSCLC细胞的侵袭。这些发现揭示了IGF2介导的自噬在CAFs活化中起着关键作用,并表明成纤维细胞中的IGF2自噬级联可能是肺癌治疗的潜在靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


