Fabrication and electrochemical investigations of nanostructured PtSn-NiTiO3 heterostructured electrocatalysts for direct methanol oxidation
IF 2
3区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
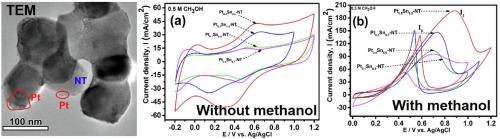
The methanol oxidation reaction of a PtSn nanoparticle decorated NiTiO3 nanostructured material system is reported in this paper. NiTiO3 and PtSn nanoparticles were formed in rhombohedral and cubic phases, respectively. The crystallite size of the PtSn nanoparticles was calculated to be 2.8 nm from the XRD peak parameters and TEM studies. The surface plasmon resonance peak observed at 308 nm indicates the decoration of PtSn-NiTiO3 nanostructures with Pt nanoparticles on the surface. The chemical oxidation states investigated by XPS showed a metallic state of Pt and Sn with a small amount of surface oxidization. From the electrochemical analysis, the Pt0.5Sn0.5-NiTiO3 nanostructures showed superior electrocatalytic performance with higher electrochemical surface area (252 m2/g), high current density (196 mA/cm2), lower Tafel value (161.31 mV/dec) and small charge transfer resistance. This work demonstrated that the Pt0.5Sn0.5 −NiTiO3 nanostructure would be a suitable electrocatalyst for oxidation for methanol in DMFC.
用于直接甲醇氧化的纳米结构 PtSn-NiTiO3 异质结构电催化剂的制备和电化学研究
本文报告了铂硒纳米粒子装饰的 NiTiO3 纳米结构材料体系的甲醇氧化反应。NiTiO3 和 PtSn 纳米粒子分别以斜方体和立方体形式形成。根据 XRD 峰参数和 TEM 研究,计算出 PtSn 纳米粒子的结晶尺寸为 2.8 nm。在 308 nm 处观察到的表面等离子共振峰表明,PtSn-NiTiO3 纳米结构的表面装饰有铂纳米粒子。通过 XPS 调查的化学氧化态显示,铂和锡处于金属态,表面有少量氧化。从电化学分析来看,Pt0.5Sn0.5-NiTiO3 纳米结构具有较高的电化学比表面积(252 m2/g)、较高的电流密度(196 mA/cm2)、较低的塔菲尔值(161.31 mV/dec)和较小的电荷转移电阻,显示出卓越的电催化性能。这项研究表明,Pt0.5Sn0.5 -NiTiO3 纳米结构将成为 DMFC 中甲醇氧化的合适电催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
4.60
自引率
4.30%
发文量
278
审稿时长
39 days
期刊介绍:
Chemical Physics publishes experimental and theoretical papers on all aspects of chemical physics. In this journal, experiments are related to theory, and in turn theoretical papers are related to present or future experiments. Subjects covered include: spectroscopy and molecular structure, interacting systems, relaxation phenomena, biological systems, materials, fundamental problems in molecular reactivity, molecular quantum theory and statistical mechanics. Computational chemistry studies of routine character are not appropriate for this journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


