Structural analysis of gum arabic side chains from Acacia seyal released by bifidobacterial β-arabino-oligosaccharide 3-O-β-l-arabinopyranosyl-α-l-arabinofuranosidase
IF 10.7
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
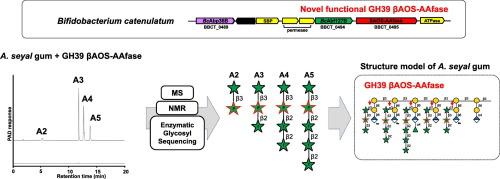
Gum arabic is widely used in the food and beverage industries for its emulsifying, stabilizing, and prebiotic effects, which promote Bifidobacterium growth. The two commercially approved varieties of gum arabic, namely, Acacia senegal gum and A. seyal gum, predominantly consist of arabinogalactan protein (AGP), albeit with different side chain modifications. We previously characterized two enzymes belonging to glycoside hydrolase (GH) family 39 in bifidobacteria involved in the release of α-D-Gal-(1→3)-α-l-Ara and β-l-Arap-(1→3)-α-l-Ara from the side chains of A. senegal gum. Although the carbohydrate structure of A. senegal gum is being increasingly explored, limited information is available on A. seyal gum. In this study, we discovered a novel GH39 β-arabino-oligosaccharide 3-O-β-l-arabinopyranosyl-α-l-arabinofuranosidase from Bifidobacterium catenulatum and revealed the accurate structure of β-l-arabino-oligosaccharides released from A. seyal gum as [β-l-Araf-(1→2)-]n-β-l-Arap-(1→3)-α-l-Araf-(1→) (n = 0–3). Growth assays and intracellular enzyme activity assessments using B. catenulatum revealed that β-l-arabino-oligosaccharides were degraded to l-arabinose by GH127 β-l-arabinofuranosidase and GH36 β-l-arabinopyranosidase. This study provides new insights into the diversity of AGP structures and the utilization mechanisms of A. seyal gum in bifidobacteria.
双歧杆菌 3-O-β-larabinopyranosyl-α-larabinofuranosidase 释放的阿拉伯胶侧链的结构分析
阿拉伯树胶具有乳化、稳定和益生作用,可促进双歧杆菌的生长,因此被广泛应用于食品和饮料行业。两种经商业批准的阿拉伯胶品种,即塞内加尔相思树胶和塞亚尔相思树胶,主要由阿拉伯半乳聚糖蛋白(AGP)组成,尽管侧链修饰不同。我们之前鉴定了双歧杆菌中属于糖苷水解酶(GH)家族 39 的两种酶,它们参与了从阿拉伯塞内加尔树胶侧链释放 α-D-Gal-(1→3)-α-l-Ara 和 β-l-Arap-(1→3)-α-l-Ara 的过程。尽管人们对塞内加尔树胶碳水化合物结构的研究越来越多,但有关塞内加尔树胶的信息却很有限。在这项研究中,我们从双歧杆菌(Bifidobacterium catenulatum)中发现了一种新型的 GH39 β-阿拉伯寡糖 3-O-β-larabinopyranosyl-α-larabinofuranosidase,并揭示了从 A. seyal 树胶中释放出的β-阿拉伯寡糖的准确结构为 [β-l-阿拉伯寡糖]。(1→2)-]n-β-l-Arap-(1→3)-α-l-Araf-(1→) (n = 0-3)。使用 B. catenulatum 进行的生长测定和细胞内酶活性评估显示,β-l-阿拉伯寡糖被 GH127 β-l-阿拉伯呋喃糖苷酶和 GH36 β-l-阿拉伯吡喃糖苷酶降解为 l-阿拉伯糖。这项研究为了解双歧杆菌中A. seyal 胶的 AGP 结构多样性和利用机制提供了新的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbohydrate Polymers
化学-高分子科学
CiteScore
22.40
自引率
8.00%
发文量
1286
审稿时长
47 days
期刊介绍:
Carbohydrate Polymers stands as a prominent journal in the glycoscience field, dedicated to exploring and harnessing the potential of polysaccharides with applications spanning bioenergy, bioplastics, biomaterials, biorefining, chemistry, drug delivery, food, health, nanotechnology, packaging, paper, pharmaceuticals, medicine, oil recovery, textiles, tissue engineering, wood, and various aspects of glycoscience.
The journal emphasizes the central role of well-characterized carbohydrate polymers, highlighting their significance as the primary focus rather than a peripheral topic. Each paper must prominently feature at least one named carbohydrate polymer, evident in both citation and title, with a commitment to innovative research that advances scientific knowledge.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


