Astragalus polysaccharides alleviate DSS-induced ulcerative colitis in mice by restoring SCFA production and regulating Th17/Treg cell homeostasis in a microbiota-dependent manner
IF 12.5
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Natural polysaccharides from Astragalus membranaceus have been shown to relieve ulcerative colitis (UC). However, the mechanism and causal relationship between the gut microbiota and Astragalus polysaccharides (APS) treatment of UC are unclear. The results of the present study showed that APS ameliorated colonic injury and the disruption of the gut microbiota and restored intestinal immune homeostasis in mice with DSS-induced colitis. Meanwhile, we found that APS treatment was ineffective in antibiotic-treated colitis mice but was effective when FMT (Fecal microbiota transplantation) was performed on UC mice using APS-treated mice as donors. APS increased the proportion of relevant microbiota that produce SCFAs and both direct administration of APS and administration of APS-adjusted gut microbiota significantly promoted the production of SCFAs in colitis mice. We demonstrated that APS dually inhibited NF-κB activation via the TLR4 and HDAC3 pathways and improved the balance in Th17/Treg cells in UC mice. In conclusion, our study revealed that APS is a promising prebiotic agent for the maintenance of intestinal health and demonstrated that APS may ameliorate colitis in a gut microbiota-dependent manner.
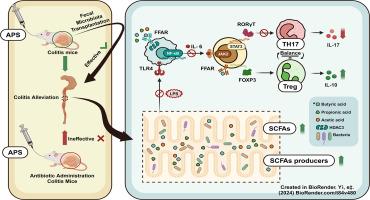
黄芪多糖以微生物群依赖的方式恢复 SCFA 的产生并调节 Th17/Treg 细胞的平衡,从而缓解 DSS 诱导的小鼠溃疡性结肠炎
从黄芪中提取的天然多糖已被证明可以缓解溃疡性结肠炎(UC)。然而,肠道微生物群与黄芪多糖(APS)治疗溃疡性结肠炎的机制和因果关系尚不清楚。本研究结果表明,APS能改善DSS诱导的小鼠结肠损伤和肠道微生物群的破坏,并恢复肠道免疫平衡。同时,我们发现 APS 治疗对抗生素治疗的结肠炎小鼠无效,但以 APS 治疗过的小鼠为供体对 UC 小鼠进行粪便微生物群移植(FMT)则有效。APS 增加了产生 SCFAs 的相关微生物群的比例,直接给予 APS 和给予经过 APS 调整的肠道微生物群都能显著促进结肠炎小鼠体内 SCFAs 的产生。我们的研究表明,APS 可通过 TLR4 和 HDAC3 途径双重抑制 NF-κB 的激活,并改善 UC 小鼠 Th17/Treg 细胞的平衡。总之,我们的研究揭示了 APS 是一种很有前景的益生元制剂,可用于维护肠道健康,并证明 APS 可通过依赖肠道微生物群的方式改善结肠炎。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbohydrate Polymers
化学-高分子科学
CiteScore
22.40
自引率
8.00%
发文量
1286
审稿时长
47 days
期刊介绍:
Carbohydrate Polymers stands as a prominent journal in the glycoscience field, dedicated to exploring and harnessing the potential of polysaccharides with applications spanning bioenergy, bioplastics, biomaterials, biorefining, chemistry, drug delivery, food, health, nanotechnology, packaging, paper, pharmaceuticals, medicine, oil recovery, textiles, tissue engineering, wood, and various aspects of glycoscience.
The journal emphasizes the central role of well-characterized carbohydrate polymers, highlighting their significance as the primary focus rather than a peripheral topic. Each paper must prominently feature at least one named carbohydrate polymer, evident in both citation and title, with a commitment to innovative research that advances scientific knowledge.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


