Staphylococcus aureus induced trained immunity in macrophages confers heterologous protection against gram-negative bacterial infection
IF 4.6
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
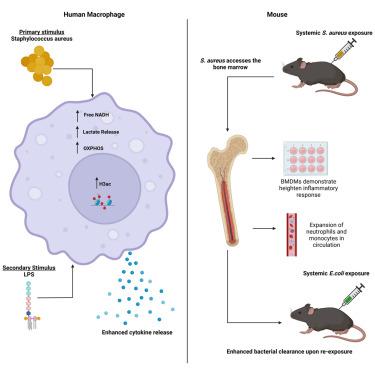
Staphylococcus aureus can induce trained immunity in murine macrophages offering protection against repeat exposure during S. aureus skin infection. Here we demonstrate that S. aureus exposure can result in non-specific trained immunity in humans and mice, enhancing macrophage responsiveness and bacterial clearance in a heterologous challenge. In humans, the enhanced macrophage responsiveness was accompanied by metabolic changes and histone modification. In mice, the enhanced responsiveness of macrophages occurred in conjunction with enhanced myelopoiesis. This report provides further insights on the host’s response to the bacterium S. aureus, indicating that exposure to this organism induces heterologous protection against subsequent gram-negative infection that is provided by macrophages. These findings support the hypothesis that S. aureus has evolved to develop a mutualistic relationship with the host, imbuing the host with enhanced capacity to protect itself from attack by alternative pathogens, while potentially allowing S. aureus to exert its dominance within its niche.
金黄色葡萄球菌诱导的巨噬细胞训练有素的免疫力可在革兰氏阴性菌感染时提供异源保护
金黄色葡萄球菌可诱导小鼠巨噬细胞产生训练有素的免疫力,从而在金黄色葡萄球菌皮肤感染时防止重复暴露。我们在这里证明,金黄色葡萄球菌暴露可导致人类和小鼠产生非特异性训练免疫,在异源挑战中增强巨噬细胞的反应能力和细菌清除能力。在人体内,巨噬细胞反应性的增强伴随着新陈代谢的变化和组蛋白的修饰。在小鼠中,巨噬细胞反应能力的增强与骨髓造血功能的增强同时发生。该报告进一步揭示了宿主对金黄色葡萄球菌的反应,表明暴露于这种病菌会诱导巨噬细胞提供异源保护,防止随后的革兰氏阴性菌感染。这些发现支持了这样一种假设,即金黄色葡萄球菌在进化过程中与宿主形成了一种互利关系,使宿主具有更强的能力保护自身免受其他病原体的攻击,同时有可能使金黄色葡萄球菌在其生态位中发挥主导作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


