Investigating the non-covalent interactions between rutin and superoxide dismutase: Focus on thermal stability, structure, and in vitro digestion
IF 6
1区 农林科学
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
Rutin is prone to degradation during processing, highlighting the need for a suitable protein system for its delivery. This study evaluated the effects of pH and concentration ratios on the interaction between rutin and superoxide dismutase (SOD), aiming to develop a suitable non-covalent delivery system. The highest thermal stability was observed at pH 4 with a rutin-to-SOD concentration ratio of 5:1, where the melting temperature (Tm) reached 67.8 °C, and the half-life was 176 min. Infrared spectroscopy and particle size analysis revealed that rutin has minimal impact on the secondary structure of SOD; however, high concentrations of rutin influence the quaternary structure of SOD, leading to aggregation. Molecular docking and molecular dynamics simulations further confirmed that the interaction between rutin and SOD occurs through hydrogen bonding and hydrophobic interactions, with pH altering their binding sites. At pH 5 and pH 6, the digestive stability of the SOD-rutin complex was the highest. This study provides a theoretical basis for the preparation of highly stable SOD-rutin complexes and identifies a new protein system for the stabilization of rutin.
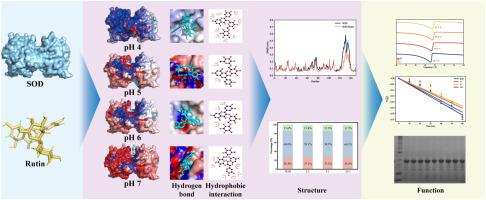
研究芦丁和超氧化物歧化酶之间的非共价相互作用:关注热稳定性、结构和体外消化
芦丁在加工过程中容易降解,因此需要一种合适的蛋白质系统来输送芦丁。本研究评估了 pH 值和浓度比对芦丁和超氧化物歧化酶(SOD)之间相互作用的影响,旨在开发一种合适的非共价递送系统。在 pH 值为 4、芦丁与 SOD 的浓度比为 5:1 时,热稳定性最高,熔化温度(Tm)达到 67.8 ℃,半衰期为 176 分钟。红外光谱和粒度分析表明,芦丁对 SOD 二级结构的影响极小;然而,高浓度的芦丁会影响 SOD 的四元结构,导致 SOD 聚合。分子对接和分子动力学模拟进一步证实,芦丁和 SOD 之间的相互作用是通过氢键和疏水作用发生的,pH 值会改变它们的结合位点。在 pH 值为 5 和 6 时,SOD-芦丁复合物的消化稳定性最高。这项研究为制备高度稳定的 SOD 芦丁复合物提供了理论依据,并为稳定芦丁确定了一种新的蛋白质系统。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

LWT - Food Science and Technology
工程技术-食品科技
CiteScore
11.80
自引率
6.70%
发文量
1724
审稿时长
65 days
期刊介绍:
LWT - Food Science and Technology is an international journal that publishes innovative papers in the fields of food chemistry, biochemistry, microbiology, technology and nutrition. The work described should be innovative either in the approach or in the methods used. The significance of the results either for the science community or for the food industry must also be specified. Contributions written in English are welcomed in the form of review articles, short reviews, research papers, and research notes. Papers featuring animal trials and cell cultures are outside the scope of the journal and will not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


