Enhancing photocatalytic H2 evolution of Cd0.5Zn0.5S with the synergism of amorphous CoS cocatalysts and surface S2− adsorption
IF 6.7
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
Abstract
Designing surface phase is an efficient strategy to facilitate charge separation and photocatalytic H2-evolution performance. In this work, CoS cocatalysts were intimately anchored on Cd0.5Zn0.5S (denoted as CZS) photocatalyst via in-situ precipitate transformation in S2−/SO32− solution with cobaltous phosphate (CoPi) as a precursor, meanwhile, S2− ions were adsorbed on the CZS to form a sulfur-rich surface (denoted as CZS-S). The photocatalytic H2-evolution rate of CoS/CZS-S is 2.02 mmol·g−1·h−1 in 0.1 M Na2S/Na2SO3 sacrificial agent system. In addition, CoS/CZS-S exhibits excellent stability in both Na2S/Na2SO3 and lactic acid system. The theoretical calculations (DFT) and experimental results reveal that amorphous CoS can work as a highly effective cocatalyst for H2 evolution reaction and the intimate contact between CZS and CoS facilitates the photoelectrons transfer from CZS to CoS. The adsorbed S2− ions mainly work as effective hole acceptors. As a result of the synergism of CoS and adsorbed S2− ions, the boosted separation and immigration of photoelectrons and photoholes and high photocatalytic H2-evolution performance of CoS/CZS-S are realized. The present work highlights simultaneous reinforcing reduction and oxidation half-reaction dynamics via a facile and economic surface strategy to achieve efficient solar H2-evolution from H2O splitting.
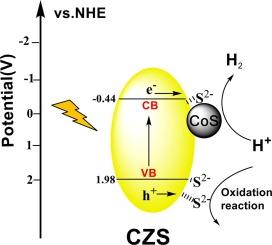
非晶 CoS 协同催化剂与表面 S2- 吸附的协同作用增强了 Cd0.5Zn0.5S 的光催化 H2 演化能力
设计表面相是促进电荷分离和光催化 H2 溶解性能的有效策略。本研究以磷酸钴(CoPi)为前驱体,在S2-/SO32-溶液中通过原位沉淀转化将CoS茧催化剂紧密锚定在Cd0.5Zn0.5S(简称CZS)光催化剂上,同时在CZS上吸附S2-离子形成富硫表面(简称CZS-S)。在 0.1 M Na2S/Na2SO3 牺牲剂体系中,CoS/CZS-S 的光催化 H2 生成率为 2.02 mmol-g-1-h-1。此外,CoS/CZS-S 在 Na2S/Na2SO3 和乳酸体系中均表现出优异的稳定性。理论计算(DFT)和实验结果表明,无定形 CoS 可作为一种高效的茧催化剂用于 H2 演化反应,CZS 和 CoS 之间的亲密接触有利于光电子从 CZS 转移到 CoS。吸附的 S2- 离子主要作为有效的空穴受体发挥作用。由于 CoS 和吸附的 S2- 离子的协同作用,CoS/CZS-S 实现了光电子和光电子孔的分离和迁移,具有很高的光催化 H2 变化性能。本研究通过一种简便、经济的表面策略,强调了同时强化还原和氧化半反应动力学,以实现高效的太阳能 H2O 裂解产生的 H2 蒸发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


