Antioxidant activity of soybean peptides and Keap1 protein: A combined in vitro and in silico analysis
IF 6
1区 农林科学
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
Antioxidant peptides can be obtained from diverse dietary protein sources with high safety. The Kelch-like ECH-associated protein 1(Keap1) is a key protein in the cellular oxidative stress signaling pathway and it plays an important role in the field of antioxidant. Soybeans are a vital source for the production of bioactive peptides. In this study, firstly, we screened 468 antioxidant peptides from soybean protein with low molecular weight, high hydrophobicity and non-toxicity. The top three antioxidant peptides were isolated and extracted from the fractions using UHPLC-QQQ/MS analysis. The free radical scavenging rate demonstrated significant antioxidant activity of PHHADS (EC50 = 4.26 mmol/L). In addition, peptide PHHADS was demonstrated significantly protects HepG2 cells from oxidative stress-induced damage. Lastly, 200 ns molecular dynamics simulations were performed between the peptides and Keap1. The results revealed that PHHADS closely interacts with the active site of Keap1. Moreover, the interaction of the antioxidant peptides with Keap1 led to the disruption of the β-sheet structure of Keap1 in residues380-390, transforming into an irregularly curved structure. This particular region in Keap1 is crucial for substrates binding, suggesting a potential inhibitory mechanism by the peptides. These findings contribute to the understanding of antioxidant peptide interactions and their potential application in developing functional foods for health benefits.
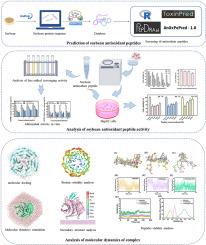
大豆肽和 Keap1 蛋白的抗氧化活性:体外和硅学综合分析
抗氧化肽可以从不同的膳食蛋白质来源中获得,安全性很高。Kelch-like ECH-associated protein 1(Keap1)是细胞氧化应激信号通路中的一个关键蛋白,在抗氧化领域发挥着重要作用。大豆是生产生物活性肽的重要来源。本研究首先从大豆蛋白中筛选出 468 种分子量小、疏水性高、无毒性的抗氧化肽。通过超高效液相色谱-质谱/质谱分析,从馏分中分离提取出抗氧化肽的前三名。自由基清除率表明 PHHADS 具有显著的抗氧化活性(EC50 = 4.26 mmol/L)。此外,多肽 PHHADS 还能显著保护 HepG2 细胞免受氧化应激引起的损伤。最后,在多肽和 Keap1 之间进行了 200 ns 的分子动力学模拟。结果显示,PHHADS 与 Keap1 的活性位点有密切的相互作用。此外,抗氧化肽与 Keap1 的相互作用导致 Keap1 在残基 380-390 处的β-片状结构被破坏,变成了不规则的弯曲结构。Keap1的这一特定区域对于底物的结合至关重要,这表明肽具有潜在的抑制机制。这些发现有助于人们了解抗氧化肽的相互作用及其在开发有益健康的功能食品中的潜在应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

LWT - Food Science and Technology
工程技术-食品科技
CiteScore
11.80
自引率
6.70%
发文量
1724
审稿时长
65 days
期刊介绍:
LWT - Food Science and Technology is an international journal that publishes innovative papers in the fields of food chemistry, biochemistry, microbiology, technology and nutrition. The work described should be innovative either in the approach or in the methods used. The significance of the results either for the science community or for the food industry must also be specified. Contributions written in English are welcomed in the form of review articles, short reviews, research papers, and research notes. Papers featuring animal trials and cell cultures are outside the scope of the journal and will not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


