Enhanced adsorption and reduction of Pb(II) from aqueous solution by sulfide-modified nanoscale zerovalent iron: characterization, kinetics and mechanisms
IF 4.4
3区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
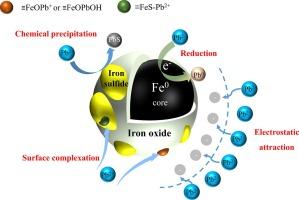
Nanoscale zerovalent iron (nZVI) was prone to aggregation and passivation, which limited its application. Here, sulfide-modified nZVI (S-nZVI) was synthesized to enhance Pb(II) removal, the transmission electron microscope and scanning electron microscope showed S-nZVI particles exhibited a core–shell structure, with Fe0 comprising the core and iron oxides in the shell, the generated iron sulfides (FeSx) were distributed over the iron oxides shell. The water contact angle results suggested S-nZVI was more hydrophobic than nZVI, which could prevent the oxidation of the inner Fe0 core with aqueous solution, furthermore, the electrochemical experiments demonstrated the FeSx improved the electron transfer efficiency from Fe0 core to contaminants, enhancing the reactivity of S-nZVI. Mechanism analysis confirmed that the electrostatic attraction, chemical precipitation, surface complexation and reduction occurred during the adsorption. S-nZVI with S/Fe molar ratio of 0.3 offered the best Pb(II) adsorption capacity, and higher pH value was favourable, the presence of co-existing cations (K+, Na+, Ca2+, Mg2+) all interfered the Pb(II) removal, followed as Ca2+ >Mg2+ > K+ > Na+. The pseudo-second-order kinetics and Langmuir model could well described the adsorption process, indicating that monolayer adsorption dominated the process. Thermodynamic results showed the adsorption was a spontaneous endothermic process, even after 4 cycles of recycling, the removal rate remained at 76.4 %, demonstrating S-nZVI had good reusability.
硫化物修饰的纳米级零价铁对水溶液中铅(II)的吸附和还原作用增强:表征、动力学和机制
纳米级零价铁(nZVI)容易聚集和钝化,限制了其应用。透射电子显微镜和扫描电子显微镜显示,S-nZVI 颗粒呈现核壳结构,核心为 Fe0,外壳为铁氧化物,生成的硫化铁(FeSx)分布在铁氧化物外壳上。水接触角结果表明,S-nZVI 比 nZVI 更疏水,这可以防止内部 Fe0 核心被水溶液氧化,此外,电化学实验表明,FeSx 提高了从 Fe0 核心到污染物的电子传递效率,增强了 S-nZVI 的反应活性。机理分析表明,吸附过程中发生了静电吸引、化学沉淀、表面络合和还原作用。S/Fe 摩尔比为 0.3 的 S-nZVI 对铅(II)的吸附能力最好,pH 值越高越有利,共存阳离子(K+、Na+、Ca2+、Mg2+)都会干扰铅(II)的去除,其次是 Ca2+ >Mg2+ > K+ > Na+。伪二阶动力学和 Langmuir 模型很好地描述了吸附过程,表明单层吸附占主导地位。热力学结果表明,吸附是一个自发的内热过程,即使在循环使用 4 次后,去除率仍保持在 76.4 %,这表明 S-nZVI 具有良好的重复使用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry Communications
化学-无机化学与核化学
CiteScore
5.50
自引率
7.90%
发文量
1013
审稿时长
53 days
期刊介绍:
Launched in January 1998, Inorganic Chemistry Communications is an international journal dedicated to the rapid publication of short communications in the major areas of inorganic, organometallic and supramolecular chemistry. Topics include synthetic and reaction chemistry, kinetics and mechanisms of reactions, bioinorganic chemistry, photochemistry and the use of metal and organometallic compounds in stoichiometric and catalytic synthesis or organic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


