Structure-based design and synthesis of (E)-l-(s-pheny1)-N-(4-(2,2,4-trimethy1–2,3-dihydro-lH-benzo[b][l,4]diazepin-l-yl)phenyl)methanimine motifs as antimicrobial and anti-tubercular agents
IF 2.7
Q2 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
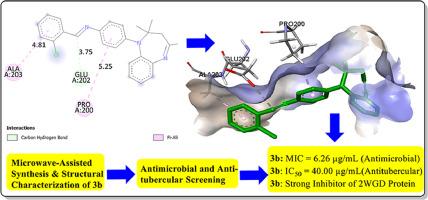
Benzodiazepines' chemistry and synthesis as heterocyclic compounds have recently attracted a lot of attention, due to their extensive biological diversity in drug design and potential for usage in agrochemicals. Eco-friendly and highly efficient method was herein reported for the synthesis of a new series of Schiff base of benzodiazepine derivatives 3a-l using microwave-assisted approach. Firstly, 2,2,4-trimethy1–2,3-dihydro-lH-benzo[b][l,4]diazepine (1) was synthesized by AgNO3-catalyzed reaction of o-phenylenediamine with excess of acetone. Coupling of benzodiazepine 1 with 4-chloroaniline afforded intermediate benzodiazepine 2 which was subsequently reacted with benzaldehyde derivatives via microwave irradiation technique to access twelve final targeted benzodiazepine Schiff bases, 3a-l. The chemical structures of the scaffolds 3a-l were authenticated using analytical and spectroscopic data. Benzodiazepine Schiff bases 3a-l were investigated for their in vitro antimicrobial activities using Agar diffusion technique and screened for their minimum inhibitory concentration (MIC) using microtube dilution technique. Ten pathogenic organisms comprising of seven bacterial and three fungal isolates were utilized for the screening. Ciprofloxacin was the positive control for antibacterial screening while fluconazole was engaged as the positive control for the antifungal screening. The most efficacious antimicrobial agent among the series was (E)-l-(2-Chloropheny1)-N-(4-(2,2,4-trimethyl-2,3-dihydro-lH-benzo[b][l,4] diazepinlyl)phenyl)methanimi ne (3b) with a MIC value of 3.13 µg/mL and MBC of 6.25 µg/mL among all the synthesized compounds synthesized and screening for antimicrobial assessment. Compound 3b also emerged as the best anti-tubercular agent IC50 of 40 µg/mL against Mycobacterium tuberculosis, Mycobacterium bovis and H37Rv
基于结构设计和合成 (E)-l-(s-pheny1)-N-(4-(2,2,4- 三甲基 1-2,3-二氢-lH-苯并[b][l,4]二氮杂卓-l-基)苯基)甲亚胺基团作为抗菌剂和抗结核剂
由于苯并二氮杂环类化合物在药物设计中具有广泛的生物多样性以及在农用化学品中的潜在用途,其化学和合成最近引起了广泛关注。本文报道了利用微波辅助方法合成苯并二氮杂卓衍生物希夫碱新系列 3a-l 的环保高效方法。首先,通过 AgNO3 催化邻苯二胺与过量丙酮的反应合成了 2,2,4-三甲基-1-2,3-二氢-lH-苯并[b][l,4]二氮杂卓(1)。苯并二氮杂卓 1 与 4-氯苯胺偶联得到中间体苯并二氮杂卓 2,然后通过微波辐照技术与苯甲醛衍生物反应,最终得到 12 个目标苯并二氮杂卓席夫碱 3a-l。利用分析和光谱数据对支架 3a-l 的化学结构进行了鉴定。使用琼脂扩散技术研究了苯并二氮杂卓席夫碱 3a-l 的体外抗菌活性,并使用微管稀释技术筛选了其最低抑菌浓度 (MIC)。筛选采用了 10 种病原体,包括 7 种细菌和 3 种真菌分离物。环丙沙星是抗菌筛选的阳性对照,而氟康唑则是抗真菌筛选的阳性对照。在合成的所有化合物中,最有效的抗菌剂是(E)-l-(2-氯苯基1)-N-(4-(2,2,4-三甲基-2,3-二氢-lH-苯并[b][l,4] 二氮杂环庚烯基)苯基)甲亚胺(3b),其 MIC 值为 3.13 µg/mL,MBC 为 6.25 µg/mL。化合物 3b 还是抗结核分枝杆菌、牛分枝杆菌和 H37Rv 的最佳抗结核剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Scientific African
Multidisciplinary-Multidisciplinary
CiteScore
5.60
自引率
3.40%
发文量
332
审稿时长
10 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


