Use of a hydrosilylation reaction for the preparation of structure-controlled boroxine-based polyborosiloxanes
IF 4.5
3区 工程技术
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
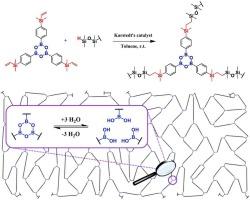
The structure of organosilicon vinyl-containing boroxine was confirmed by 1H nuclear magnetic resonance (NMR) spectroscopy, infrared (IR) spectroscopy and matrix-assisted laser desorption/ionization (MALDI) mass spectrometry. Its thermal properties were studied using thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) methods. The ability of boroxine to undergo hydrosilylation using the Karstedt's catalyst was investigated on various organosilicon substrates. Boroxine was shown to be involved in these reactions as a cross-linking agent. The formation of dynamic boroxine cross-links allowed the analysis of the reaction products by NMR spectroscopy, including the use of polyfunctional hydride-containing reagents. In all cases the addition proceeds selectively to the β-position and with complete conversion. The structure of the resulting polyborosiloxane containing 1.4 mol% of modified units was also confirmed by 1H NMR spectroscopy, and its thermal and rheological properties were studied.
利用氢硅烷化反应制备结构受控的硼氧烷基聚硼硅氧烷
通过 1H 核磁共振(NMR)光谱、红外光谱和基质辅助激光解吸/电离(MALDI)质谱法确认了含乙烯基硼氧有机硅的结构。使用热重分析法(TGA)和差示扫描量热法(DSC)对其热性质进行了研究。研究了硼氧烷在各种有机硅基质上使用卡氏催化剂进行氢化硅的能力。研究表明,硼氧烷作为一种交联剂参与了这些反应。由于形成了动态硼氧烷交联,因此可以通过核磁共振光谱分析反应产物,包括使用含多官能团氢化物的试剂。在所有情况下,加成反应都选择性地进行到 β 位,并且完全转化。此外,还通过 1H NMR 光谱证实了所得到的含有 1.4 摩尔%改性单元的聚硼硅氧烷的结构,并研究了其热学和流变学特性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Reactive & Functional Polymers
工程技术-高分子科学
CiteScore
8.90
自引率
5.90%
发文量
259
审稿时长
27 days
期刊介绍:
Reactive & Functional Polymers provides a forum to disseminate original ideas, concepts and developments in the science and technology of polymers with functional groups, which impart specific chemical reactivity or physical, chemical, structural, biological, and pharmacological functionality. The scope covers organic polymers, acting for instance as reagents, catalysts, templates, ion-exchangers, selective sorbents, chelating or antimicrobial agents, drug carriers, sensors, membranes, and hydrogels. This also includes reactive cross-linkable prepolymers and high-performance thermosetting polymers, natural or degradable polymers, conducting polymers, and porous polymers.
Original research articles must contain thorough molecular and material characterization data on synthesis of the above polymers in combination with their applications. Applications include but are not limited to catalysis, water or effluent treatment, separations and recovery, electronics and information storage, energy conversion, encapsulation, or adhesion.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


