The role of multivalent cations in determining the cross-linking affinity of alginate hydrogels: A combined experimental and modeling study
IF 5.5
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
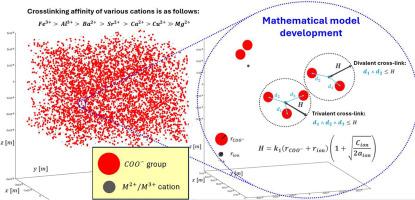
This study explores the development of a comprehensive mathematical model to predict the cross-link density of alginate hydrogels using varying concentrations of divalent and trivalent ions. By systematically investigating the effects of Ca²⁺, Ba²⁺, Cu²⁺, Sr²⁺, Mg²⁺, Fe3⁺ and Al³⁺, the research describes the relationship between ion concentration and cross-link density. Experimental data reveals that the type and concentration of these ions critically influence the mechanical properties of the resulting hydrogels, with trivalent ions such as Fe³⁺ forming stronger, triple cross-links that significantly enhance the hydrogel's mechanical strength. Among the divalent ions, the trend in binding affinity is as follows: Ba²⁺ with the highest affinity followed by Sr²⁺, Ca²⁺ and Cu²⁺, while Mg²⁺ stands out with the lowest affinity, significantly differing from the others. The deviation of Cu²⁺ (transition metal ion) from the expected trend in ion interactions suggests that coordination chemistry, along with ionic radius, valence, and cation coordination abilities, plays a significant role in determining interaction strength with alginate. The proposed model, enhanced with fitting parameters k1 and k2 to account for ion-specific effects, leverages the unique binding affinities and coordination chemistry of each ion to tailor alginate hydrogels for specific applications. The parameter k1 reflects the affinity of the ions for the alginate chains, while k2 captures the coordination abilities and cross-linking efficiency. This work not only advances the understanding of ion-mediated cross-linking in alginate systems but also offers a valuable tool for the design and optimization of hydrogels with precise mechanical properties governed by various applications.
多价阳离子在决定海藻酸盐水凝胶交联亲和力中的作用:实验与建模相结合的研究
本研究探索建立一个综合数学模型,以预测使用不同浓度的二价和三价离子时海藻酸盐水凝胶的交联密度。通过系统研究 Ca²⁺、Ba²⁺、Cu²⁺、Sr²⁺、Mg²⁺、Fe3⁺ 和 Al³⁺ 的影响,研究描述了离子浓度与交联密度之间的关系。实验数据显示,这些离子的类型和浓度对所制备水凝胶的机械性能有着至关重要的影响,三价离子(如 Fe³⁺)会形成更强的三重交联,从而显著提高水凝胶的机械强度。在二价离子中,结合亲和力的变化趋势如下:Ba²⁺的亲和力最高,其次是Sr²⁺、Ca²⁺和Cu²⁺,而Mg²⁺的亲和力最低,与其他离子有显著差异。Cu²⁺(过渡金属离子)偏离了离子相互作用的预期趋势,这表明配位化学以及离子半径、价和阳离子配位能力在决定与海藻酸盐的相互作用强度方面起着重要作用。所提出的模型利用拟合参数 k1 和 k2 增强了离子特异性效应,充分利用了每种离子独特的结合亲和力和配位化学性质,为特定应用定制了海藻酸盐水凝胶。参数 k1 反映了离子与海藻酸盐链的亲和力,而 k2 则反映了配位能力和交联效率。这项工作不仅加深了人们对海藻酸盐体系中离子介导的交联的理解,还为设计和优化具有各种应用所需的精确机械性能的水凝胶提供了宝贵的工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal Advances
Engineering-Industrial and Manufacturing Engineering
CiteScore
8.30
自引率
0.00%
发文量
213
审稿时长
26 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


