Rubisco at interfaces II: Structural reassembly enhances oil-water interface and emulsion stabilization
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Rubisco is the most abundant protein on earth and has gained extensive attentions as a novel food ingredient, such as an emulsifier. Extraction methods can significantly affect its molecular structures and consequently influence its oil-water interface and emulsion stabilization properties. This work aims to elucidate the role of the Rubisco molecular structure in stabilizing the oil-water interface and the multiphase system of emulsions. Ultrafiltration (mild) and acid precipitation-alkaline redispersion (extensive) were used to extract Rubisco from spinach leaves. Protein molecular properties were characterized by size exclusion chromatography (SEC), circular dichroism (CD), and fluorescence spectrometry. Subsequently, the oil-water interfacial properties, including the adsorption and rheological behavior in both small and large dilatational and shear deformations, and the emulsion stabilization properties of Rubisco were investigated. We found that acid precipitation-alkaline redispersion produced a Rubisco extract (RA) with extensive structural reassembling, compared to the one produced by ultrafiltration (RU), for which nativity was mostly retained. RA had two-fold higher surface hydrophobicity than RU, and this caused RA to adsorb faster to the oil-water interface and developed a stiffer solid-like interface (Gi’ = 26 ± 3 mN/m) than RU (Gi’ = 15 ± 2 mN/m), which was also more resistant to density changes in large dilatational deformations. Consequently, RA displayed higher emulsifying activity and emulsion stability to coalescence during bulk shear and storage. Additionally, structural reassembly resulted in a higher value of the zeta potential of RA, which made the emulsion more stable against flocculation, compared to RU. Our study demonstrates that structural reassembly might be a useful strategy to improve the behavior of plant proteins in oil-water interface and emulsion stabilization, and may stimulate the development of new plant protein-stabilized emulsion-based products.
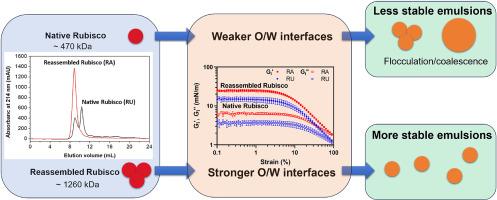
界面上的 Rubisco II:结构重组增强了油水界面和乳液的稳定性
Rubisco 是地球上最丰富的蛋白质,作为乳化剂等新型食品配料受到广泛关注。萃取方法会严重影响其分子结构,进而影响其油水界面和乳液稳定特性。本研究旨在阐明 Rubisco 分子结构在稳定油水界面和乳剂多相体系中的作用。研究人员采用超滤(温和)和酸沉淀-碱性再分散(广泛)方法从菠菜叶中提取 Rubisco。通过尺寸排阻色谱法(SEC)、圆二色光谱法(CD)和荧光光谱法对蛋白质的分子特性进行了表征。随后,研究了 Rubisco 的油水界面特性,包括在大小扩张和剪切变形中的吸附和流变行为,以及乳液稳定特性。我们发现,酸沉淀-碱性再分散产生的 Rubisco 提取物(RA)与超滤产生的 Rubisco 提取物(RU)相比,具有广泛的结构重组,而超滤产生的 Rubisco 提取物(RU)大部分保留了原生性。与 RU(Gi' = 15 ± 2 mN/m)相比,RA 的表面疏水性比 RU 高出两倍,这使得 RA 能够更快地吸附到油水界面,并形成一个更坚硬的固态界面(Gi' = 26 ± 3 mN/m),而且在大的扩张变形过程中更能抵抗密度变化。因此,RA 显示出更高的乳化活性和乳液稳定性,在散装剪切和储存过程中不会发生凝聚。此外,与 RU 相比,结构重组使 RA 的 zeta 电位值更高,从而使乳液对絮凝更稳定。我们的研究表明,结构重组可能是改善植物蛋白在油水界面和乳液稳定中的行为的有效策略,并可能促进新的植物蛋白稳定乳液产品的开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


