Unveiling the influences of electrolyte additives on the fast-charging performance of lithium-ion batteries
IF 8.1
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Enhancing the fast-charging capability of lithium-ion batteries is a promising way to extend the driving range of electric vehicles. One of the most effective and economic ways is to regulate the electrode-electrolyte interphase chemistry by employing electrolyte additives. This requires a comprehensive understanding of the intrinsic role and effectiveness of different electrolyte additives. In this work, five common electrolyte additives are comprehensively compared in graphite | LiNi0.8Mn0.1Co0.1O2 lithium-ion cells, including lithium difluorophosphate (PFO), lithium bis(oxalato)borate (LiBOB), lithium difluoro (oxalato)borate (DFOB), tris(trimethylsilyl) phosphite (TMSPi), and tris(trimethylsilyl) phosphate (TMSPA). Although all of them are found to improve fast-charging performance over the baseline electrolyte, comprehensive analyses show that DFOB and TMSPi are the most effective additives, which results from their capability of ameliorating the electrode-electrolyte interphases at both the cathode and anode. This is confirmed with X-ray photoelectron spectroscopy and comprehensive electrochemical characterizations. In contrast, LiBOB and PFO can stabilize the cathode well, but make the anode-electrolyte interphase more resistive. Overall, this research expands the understanding of the role of electrolyte additives in fast-charging lithium-ion batteries.
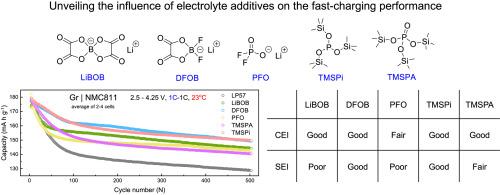
揭示电解质添加剂对锂离子电池快速充电性能的影响
提高锂离子电池的快速充电能力是延长电动汽车行驶里程的一种可行方法。最有效、最经济的方法之一是通过使用电解质添加剂来调节电解质-电解质相间化学。这就需要全面了解不同电解质添加剂的内在作用和功效。本研究全面比较了五种常用电解质添加剂在石墨 | LiNi0.8Mn0.1Co0.1O2锂离子电池中的应用进行了综合比较,包括二氟磷酸锂(PFO)、双(草酸)硼酸锂(LiBOB)、二氟(草酸)硼酸锂(DFOB)、三(三甲基硅基)亚磷酸酯(TMSPi)和三(三甲基硅基)磷酸酯(TMSPA)。虽然与基线电解质相比,所有这些添加剂都能改善快速充电性能,但综合分析表明,DFOB 和 TMSPi 是最有效的添加剂,这是因为它们能改善阴极和阳极的电极-电解质相间。X 射线光电子能谱和全面的电化学特性分析证实了这一点。相比之下,LiBOB 和 PFO 能够很好地稳定阴极,但却使阳极-电解质相间更具电阻性。总之,这项研究拓展了人们对电解质添加剂在快速充电锂离子电池中作用的认识。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


