Enhancing sodium ion transport in batteries through a crosslinked ceramic network-coated polyethylene (PE) separator
IF 8.1
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
As integral constituents of sodium ion batteries (SIBs), separator not only segregate positive and negative electrodes but also wield significant influence over electrochemical performance enhancement. Here, we present a hydroxyapatite nanowires composite separator (N-HAP@PE) by blade coating, boasting exceptional Na-ion transport capacity and high safety. The N-HAP@PE separator features a crosslinked ceramic network structure coating endowed with polar functional groups (-OH), providing excellent electrolyte wettability. Its compatibility with ester-based electrolytes promotes efficient transport of Na-ion between the cathode and anode, increasing the ion conductivity (0.215 mS cm−1) and a Na-ion transference number (0.62). Importantly, the sodiophilic HAP-NWs coating enables highly reversible Na plating and stripping while inhibiting dendrite formation. Ultimately, the N-HAP@PE separator assembled with NaNi1/3Fe1/3Mn1/3O2 cathode (areal density: 13.24 mg cm−2) and hard carbon anode (areal density: 6.3 mg cm−2), exhibits excellent capacity retention (85 %) after 200 cycles at 2C. This offers a new approach for the development of high-performance and low-cost sodium-ion batteries.
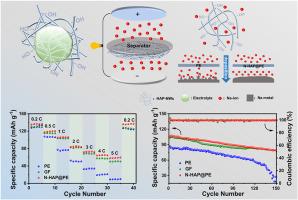
通过交联陶瓷网络涂层聚乙烯(PE)隔板增强电池中的钠离子传输
作为钠离子电池(SIB)的重要组成部分,隔膜不仅能隔离正负电极,还对电化学性能的提高具有重要影响。在这里,我们通过叶片涂层展示了一种羟基磷灰石纳米线复合隔膜(N-HAP@PE),它具有优异的钠离子传输能力和高安全性。N-HAP@PE 分离器的特点是具有极性官能团(-OH)的交联陶瓷网络结构涂层,可提供出色的电解质润湿性。它与酯基电解质的兼容性促进了 Na 离子在阴极和阳极之间的高效传输,提高了离子电导率(0.215 mS cm-1)和 Na 离子转移数(0.62)。重要的是,亲钠的 HAP-NWs 涂层能够实现高度可逆的 Na 镀层和剥离,同时抑制枝晶的形成。最终,N-HAP@PE 分离器与 NaNi1/3Fe1/3Mn1/3O2 阴极(平均密度:13.24 毫克/厘米-2)和硬碳阳极(平均密度:6.3 毫克/厘米-2)组装在一起,在 2C 温度下循环 200 次后显示出优异的容量保持率(85%)。这为开发高性能、低成本的钠离子电池提供了一种新方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


