A layer-structured high entropy oxide with highly reversible Fe3+/Fe4+ redox as advanced cathode material for sodium ion batteries
IF 8.1
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
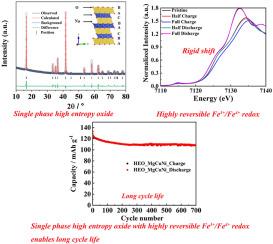
Reversible redox center is essential for long-life electrode materials. Iron is an earth abundant element, in lithium-ion batteries, highly reversible Fe2+/Fe3+ redox in LiFePO4 has play important role as redox center, Fe3+/Fe4+ redox in lithium layer-structured oxides display poor electrochemical performance. In sodium ion batteries, Fe3+/Fe4+ redox in sodium layer-structured oxides are active, while the cycle performance of Fe-contained sodium layer-structured oxide cathode need to be further improved. Herein, A pure-phase layer-structured high entropy oxide O3-Na(MgCu)1/12(NiCoFeMnTi)1/6O2 is synthesized and investigated as cathode for sodium ion battery. A reversible phase-transition takes place during the charge/discharge process. Particularly, highly reversible Fe3+/Fe4+ redox is revealed by X-ray absorption fine structure (XAFS). The as-synthesized high entropy oxide delivers a discharge capacity of 146.6 mAh g−1 at 10 mA g−1, and can retain 83.2 % of capacity after 700 cycles at 100 mA g−1 between 2.0 and 4.1 V vs. Na+/Na. In this work, Fe K-edge of Fe3+/Fe4+ redox displays rigid shift, HEO-MgCuNi could be a platform to investigate the fundamental property of Fe3+/Fe4+ redox.
一种具有高度可逆 Fe3+/Fe4+ 氧化还原作用的层状结构高熵氧化物,可作为钠离子电池的先进阴极材料
可逆氧化还原中心对长寿命电极材料至关重要。铁是地球上丰富的元素,在锂离子电池中,LiFePO4 中高度可逆的 Fe2+/Fe3+ 氧化还原作为氧化还原中心发挥着重要作用,而锂层结构氧化物中的 Fe3+/Fe4+ 氧化还原则显示出较差的电化学性能。在钠离子电池中,钠层结构氧化物中的 Fe3+/Fe4+ 氧化还原作用活跃,而含铁钠层结构氧化物正极的循环性能有待进一步提高。本文合成了一种纯相层结构高熵氧化物 O3-Na(MgCu)1/12(NiCoFeMnTi)1/6O2,并将其作为钠离子电池正极进行了研究。在充放电过程中发生了可逆相变。特别是,X 射线吸收精细结构(XAFS)揭示了高度可逆的 Fe3+/Fe4+ 氧化还原。合成的高熵氧化物在 10 mA g-1 下的放电容量为 146.6 mAh g-1,并且在 2.0 至 4.1 V 之间以 100 mA g-1 对 Na+/Na 进行 700 次循环后仍能保持 83.2% 的容量。在这项工作中,Fe3+/Fe4+ 氧化还原的 Fe K 边出现了刚性位移,HEO-MgCuNi 可作为研究 Fe3+/Fe4+ 氧化还原基本特性的平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


