Design and green synthesis of asymmetric 3,4,5-trimethoxyphenyl-inspired 1,5-diaryl-1,4-pentadien-3-ones as potent anti-gastric cancer agents through ROS and JNK-triggered apoptosis
IF 5.5
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Asymmetric 1,5-diheteroarylpenta-1,4-dien-3-one skeleton and 3,4,5-trimethoxy- phenyl group could be regarded as potent elements for designing chemotherapy agents, respectively. With the disadvantages of previous synthesis, a greener and simple procedure was developed for one-step synthesis of (E)-4(3,4,5- trimethoxypheyl)but-3-en-2-one intermediate under the Cs2CO3/EtOH/H2O system. Hence, a series of asymmetric 1,5-diaryl-1,4-pentadien-3-ones inspired 3,4,5-trimethoxyphenyl were designed, optimized synthesized and investigated the anti-gastric cancer activity. Among them, most compounds showed good anti-gastric cancer activity. The quantitative structure-activity relationship models (QSAR) of compounds were constructed through by random forest algorithm and R2 in two tumor cells were greater than 0.88. Compound 7c was elucidated to inhibit tumor growth, migration, and trigger death of SGC-7901 cells by up-regulating the generation of ROS and JNK-related apoptosis. In total, a greener system for synthesizing intermediates is provided, and compound 7c may serve as a potential chemotherapy agent for gastric cancer.
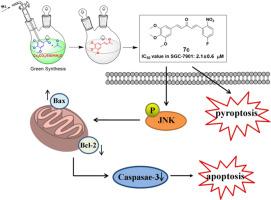
设计和绿色合成由 3,4,5-三甲氧基苯基启发的不对称 1,5-二芳基-1,4-戊二烯-3-酮,通过 ROS 和 JNK 触发细胞凋亡,作为有效的抗胃癌药物
不对称的 1,5-二杂环戊-1,4-二烯-3-酮骨架和 3,4,5-三甲氧基苯基可分别被视为设计化疗药物的有效元素。鉴于以往合成的缺点,我们开发了一种更环保、更简单的方法,在 Cs2CO3/EtOH/H2O 体系下一步合成 (E)-4(3,4,5-三甲氧基苯基)丁-3-烯-2-酮中间体。因此,我们设计、优化合成了一系列不对称的 1,5-二芳基-1,4-戊二烯-3-酮,并研究了它们的抗胃癌活性。其中,大多数化合物显示出良好的抗胃癌活性。通过随机森林算法构建了化合物的定量结构-活性关系模型(QSAR),在两个肿瘤细胞中的 R2 均大于 0.88。化合物7c通过上调ROS的生成和JNK相关的细胞凋亡,抑制了肿瘤的生长、迁移,并引发了SGC-7901细胞的死亡。总之,该研究提供了一种更绿色的中间体合成系统,化合物 7c 可作为一种潜在的胃癌化疗药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Sustainable Chemistry and Pharmacy
Environmental Science-Pollution
CiteScore
8.20
自引率
6.70%
发文量
274
审稿时长
37 days
期刊介绍:
Sustainable Chemistry and Pharmacy publishes research that is related to chemistry, pharmacy and sustainability science in a forward oriented manner. It provides a unique forum for the publication of innovative research on the intersection and overlap of chemistry and pharmacy on the one hand and sustainability on the other hand. This includes contributions related to increasing sustainability of chemistry and pharmaceutical science and industries itself as well as their products in relation to the contribution of these to sustainability itself. As an interdisciplinary and transdisciplinary journal it addresses all sustainability related issues along the life cycle of chemical and pharmaceutical products form resource related topics until the end of life of products. This includes not only natural science based approaches and issues but also from humanities, social science and economics as far as they are dealing with sustainability related to chemistry and pharmacy. Sustainable Chemistry and Pharmacy aims at bridging between disciplines as well as developing and developed countries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


