Applicability of the European Commission’s framework on safe and sustainable by design to the pharmaceutical sector
IF 5.8
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
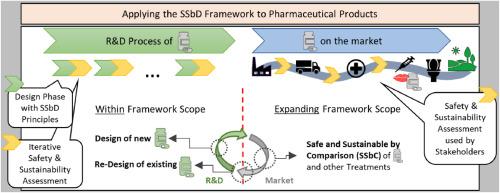
The chemical sector contributes significantly to anthropogenic impacts on planetary health. Thus, there is a need for a green transformation. The same holds for the pharmaceutical sector. To assist with this transformation, European Commission’s Joint Research Centre (JRC) developed a framework on Safe and Sustainable by Design (SSbD) for chemicals and materials (“SSbD framework”). The general structure of the SSbD framework leaves room for sector-specific priorities and practices. This study explores the applicability of the SSbD framework to the pharmaceutical sector; more specifically to the development, production and use of Human Medicinal Products (HMPs). We show that the stage-gate process in R&D of HMPs fits very well with the design-assessment process of the SSbD framework, making the framework conceptually applicable to pharmaceutical R&D. Future efforts should focus on the development of i) methods to predict environmental safety and sustainability based on the limited data available during R&D, ii) a pragmatic procedure integrating SSbD into HMPs innovation, and iii) a weighing system considering also environmental safety and sustainability parameters alongside patient safety and medical efficacy. Although the assessment phase of the JRC’s SSbD framework has specifically been developed for innovation purposes, we propose an expansion of its scope to pharmaceutical products already on the market. The reason is its applicability by healthcare actors to guide safer and more sustainable choices regarding the use of marketed HMPs. We call this approach Safe and Sustainable by Comparison (SSbC) and show that the assessment principles of the SSbD framework can be applied to SSbC.
欧盟委员会的安全和可持续设计框架对制药行业的适用性
化工行业在很大程度上造成了人类活动对地球健康的影响。因此,有必要进行绿色转型。制药行业也是如此。为协助这一转型,欧盟委员会联合研究中心(JRC)制定了化学品和材料安全与可持续设计(SSbD)框架("SSbD 框架")。SSbD 框架的总体结构为特定行业的优先事项和实践留出了空间。本研究探讨了 SSbD 框架对制药行业的适用性,更具体地说,是对人用医药产品 (HMP) 的开发、生产和使用的适用性。我们的研究表明,人类医药产品研发的阶段-关口流程与 SSbD 框架的设计-评估流程非常吻合,从而使该框架在概念上适用于医药研发。未来的工作重点应放在以下几个方面:i) 根据研发过程中的有限数据,开发预测环境安全性和可持续性的方法;ii) 将 SSbD 纳入 HMP 创新的实用程序;iii) 在考虑患者安全和医疗效果的同时,也考虑环境安全性和可持续性参数的权衡系统。虽然 JRC 的 SSbD 框架的评估阶段是专门为创新目的开发的,但我们建议将其范围扩大到已上市的医药产品。原因是该框架适用于医疗机构,可指导其在使用已上市的 HMP 时做出更安全、更可持续的选择。我们将这种方法称为 "比较安全与可持续"(SSbC),并说明 SSbD 框架的评估原则可应用于 SSbC。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Sustainable Chemistry and Pharmacy
Environmental Science-Pollution
CiteScore
8.20
自引率
6.70%
发文量
274
审稿时长
37 days
期刊介绍:
Sustainable Chemistry and Pharmacy publishes research that is related to chemistry, pharmacy and sustainability science in a forward oriented manner. It provides a unique forum for the publication of innovative research on the intersection and overlap of chemistry and pharmacy on the one hand and sustainability on the other hand. This includes contributions related to increasing sustainability of chemistry and pharmaceutical science and industries itself as well as their products in relation to the contribution of these to sustainability itself. As an interdisciplinary and transdisciplinary journal it addresses all sustainability related issues along the life cycle of chemical and pharmaceutical products form resource related topics until the end of life of products. This includes not only natural science based approaches and issues but also from humanities, social science and economics as far as they are dealing with sustainability related to chemistry and pharmacy. Sustainable Chemistry and Pharmacy aims at bridging between disciplines as well as developing and developed countries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


