Efficient separation of methyl ethyl ketone and water azeotrope using hydrophobic amino acid ester ionic liquids
IF 5.5
3区 工程技术
Q1 ENGINEERING, CHEMICAL
Journal of the Taiwan Institute of Chemical Engineers
Pub Date : 2024-11-07
DOI:10.1016/j.jtice.2024.105822
引用次数: 0
Abstract
Background
Methyl ethyl ketone (MEK), an essential organic solvent, is commonly produced via the n-butene method, where water emerges as the principal impurity. The conventional distillation processes, which are necessitated by the azeotropic behavior between MEK and water, result in a substantial expenditure of energy. As a result, liquid-liquid extraction represents a promising alternative for energy-efficient separation.
Methods
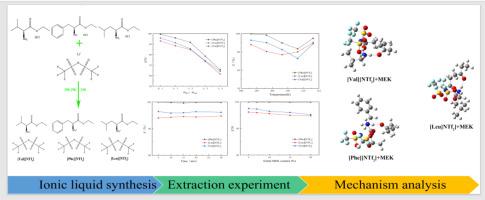
In this work, three hydrophobic amino acid ester ionic liquids L-phenylalanine ethyl ester bis(trifluoromethylsulfonyl) imide ([Phe][NTf2]), L-leucine ethyl ester bis(trifluoromethylsulfonyl) imide ([Leu][NTf2]) and L-valine ethyl ester bis(trifluoromethylsulfonyl) imide ([Val][NTf2]) were utilized as extractants for separation of the binary azeotrope methyl ethyl ketone and water. The effects of extraction time, extraction temperature, mass ratio of MEK-water mixture to ionic liquid and initial concentration of MEK on extraction efficiency were investigated.
Significant findings
The results demonstrate that the ionic liquid [Phe][NTf2] exhibits superior extraction ability for the separation of the azeotrope methyl ethyl ketone and water. The maximum extraction yield of 99.86 % was achieved with the optimum extraction conditions of extraction time, 10 min, extraction temperature, 293.15 K, mass ratio of mixture to ionic liquid, 1:1 and initial concentration of MEK, 20 %. In addition, the relationship between the structure of ionic liquids and their extraction performance was revealed by quantum chemical calculations of ionic liquids with MEK and water. These ionic liquids were positioned as promising environmentally friendly alternatives to traditional organic solvents for the recovery of MEK from aqueous solutions, providing valuable insights for industrial applications.
使用疏水性氨基酸酯离子液体高效分离甲乙酮和水共沸物
背景甲基乙基酮(MEK)是一种重要的有机溶剂,通常通过正丁烯法生产,其中水是主要的杂质。由于 MEK 与水之间的共沸行为,传统的蒸馏过程需要消耗大量能源。因此,液-液萃取是一种很有前景的节能分离替代方法。方法在这项工作中,三种疏水性氨基酸酯离子液体 L-苯丙氨酸乙酯双(三氟甲基磺酰基)亚胺([Phe][NTf2])、L-亮氨酸乙酯双(三氟甲基磺酰基)亚胺([Leu][NTf2])和L-缬氨酸乙酯双(三氟甲基磺酰基)亚胺([Val][NTf2])作为萃取剂,用于分离二元共沸物甲乙酮和水。结果表明,离子液体[Phe][NTf2]在共沸物甲乙酮和水的分离中表现出优异的萃取能力。在萃取时间为 10 分钟、萃取温度为 293.15 K、混合物与离子液体的质量比为 1:1、MEK 初始浓度为 20% 的最佳萃取条件下,萃取率最高,达到 99.86%。此外,离子液体与 MEK 和水的量子化学计算揭示了离子液体结构与其萃取性能之间的关系。这些离子液体被定位为从水溶液中回收 MEK 的传统有机溶剂的环保型替代品,为工业应用提供了宝贵的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.10
自引率
14.00%
发文量
362
审稿时长
35 days
期刊介绍:
Journal of the Taiwan Institute of Chemical Engineers (formerly known as Journal of the Chinese Institute of Chemical Engineers) publishes original works, from fundamental principles to practical applications, in the broad field of chemical engineering with special focus on three aspects: Chemical and Biomolecular Science and Technology, Energy and Environmental Science and Technology, and Materials Science and Technology. Authors should choose for their manuscript an appropriate aspect section and a few related classifications when submitting to the journal online.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


