Protonation-induced tautomerization lowers the activation barriers for N-glycosidic bond cleavage of thymidine and 5-methyluridine
IF 1.7
3区 化学
Q3 PHYSICS, ATOMIC, MOLECULAR & CHEMICAL
引用次数: 0
Abstract
Synergistic infrared multiple photon dissociation (IRMPD) action spectroscopy experiments and electronic structure calculations revealed that both 2,4-dihydroxy tautomers and O2 protonated conformers of protonated thymidine, [dThd+H]+, and protonated 5-methyluridine, [Thd+H]+ coexist in the gas phase with the 2,4-dihydroxy tautomers dominating the population. In the current study, the kinetic energy dependence of the collision-induced dissociation behavior of [dThd+H]+ and [Thd+H]+ are examined in a guided ion beam tandem mass spectrometer. The primary dissociation pathways observed involve N-glycosidic bond cleavage leading to competitive elimination of either protonated or neutral thymine. The potential energy surfaces (PESs) for these N-glycosidic bond cleavage pathways are mapped via electronic structure calculations for the mixture of 2,4-dihydroxy tautomers and O2 protonated conformers of [dThd+H]+ and [Thd+H]+ populated in the IRMPD experiments. The activation energies and heats of reaction predicted for N-glycosidic bond cleavage at the B3LYP and MP2(full) levels of theory are compared to the measured values. The agreement between experiment and theory indicates that B3LYP provides better estimates of the energetics of the species along the PESs for N-glycosidic bond cleavage of [dThd+H]+ and [Thd+H]+ than MP2, and that the 2,4-dihydroxy tautomers, which are stabilized by strong hydrogen-bonding interactions, control the threshold dissociation behavior of [dThd+H]+ and [Thd+H]+.
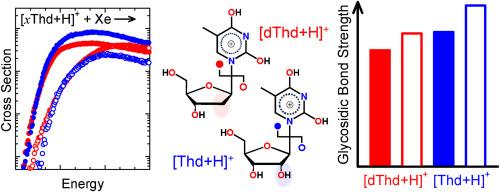
质子化诱导的同分异构降低了胸苷和 5-甲基尿苷 N-糖苷键裂解的活化障碍
协同红外多光子解离(IRMPD)作用光谱实验和电子结构计算显示,质子化胸苷[dThd+H]+和质子化 5-甲基尿苷[Thd+H]+的 2,4-二羟基同系物和 O2 质子化构象物在气相中共存,其中 2,4-二羟基同系物占多数。本研究在导引离子束串联质谱仪中考察了 [dThd+H]+ 和 [Thd+H]+ 碰撞诱导解离行为的动能依赖性。观察到的主要解离途径涉及 N-糖苷键的裂解,导致质子化或中性胸腺嘧啶的竞争性消除。通过对 IRMPD 实验中出现的 2,4-二羟基同系物和 [dThd+H]+ 和 [Thd+H]+ 的 O2 质子化构象混合物进行电子结构计算,绘制了这些 N-糖苷键裂解途径的势能面 (PES)。将 B3LYP 和 MP2(全)理论水平预测的 N-糖苷键裂解活化能和反应热与测量值进行了比较。实验与理论之间的一致表明,B3LYP 比 MP2 更好地估算了 [dThd+H]+ 和 [Thd+H]+ N-糖苷键裂解时沿 PES 的物种能量,而且通过强氢键相互作用稳定的 2,4- 二羟基同系物控制了 [dThd+H]+ 和 [Thd+H]+ 的阈值解离行为。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.60
自引率
5.60%
发文量
145
审稿时长
71 days
期刊介绍:
The journal invites papers that advance the field of mass spectrometry by exploring fundamental aspects of ion processes using both the experimental and theoretical approaches, developing new instrumentation and experimental strategies for chemical analysis using mass spectrometry, developing new computational strategies for data interpretation and integration, reporting new applications of mass spectrometry and hyphenated techniques in biology, chemistry, geology, and physics.
Papers, in which standard mass spectrometry techniques are used for analysis will not be considered.
IJMS publishes full-length articles, short communications, reviews, and feature articles including young scientist features.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


