Unveiling the promotional mechanisms of N-doping on the adsorption behaviors of dioxins from sintering flue gas by coconut shell-derived hierarchical porous carbon
IF 6.7
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
Abstract
The heteroatom doping into the activated carbon (AC) has been proved to be one of powerful tools to remove dioxins from sintering flue gas. However, the fact that the intrinsic enhanced mechanism of specific nitrogen species still remains unanswered makes the choice and design of AC suitable for dioxins elimination difficult. Herein, nitrogen-doping AC with alterable N species were prepared by melamine modification to in-depth illuminate the promotional roles of N-containing groups on the adsorption of chlorobenzene (CB) (a model compound for dioxins) over ACs through experimental and density functional theory (DFT). The results demonstrated that the N-doping was obviously conducive to CB adsorption, and pyrrole N group with the strongest adsorption energy (−0.84 eV) between AC-pyrrole and CB was determined to be the key adsorption sites for CB. ACM600 with more pyrrole N group prominently improved the chemical adsorption of total adsorption amount from 8.18 % to 15.12 %. The adsorption mechanism of CB onto ACs was governed by physical adsorption and weak chemical adsorption, which was attributed to the synergistic effects of π-π stacking interaction and hydrogen bonds, and the π-π stacking interaction dominated the adsorption interactions. The introduction of heteroatom N enhanced the adsorption capacity by promoting the chemical reactivity and π-electron density distribution of AC, and forming more significant π-π stacking interaction with the π-acceptor. The study provided a sound theoretical guideline and scientific foundation for the design and estimation of carbonaceous materials for dioxins abatement.
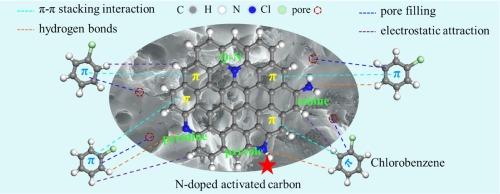
揭示椰壳衍生分层多孔碳掺杂氮对烧结烟气中二恶英吸附行为的促进机制
事实证明,在活性炭(AC)中掺杂杂原子是去除烧结烟气中二恶英的有力工具之一。然而,特定氮物种的内在增强机理仍未得到解答,这给选择和设计适用于消除二恶英的活性炭带来了困难。本文通过三聚氰胺改性制备了具有可改变氮物种的氮掺杂 AC,并通过实验和密度泛函理论(DFT)深入揭示了含氮基团对 AC 吸附氯苯(CB)(二恶英的模型化合物)的促进作用。结果表明,N掺杂明显有利于CB的吸附,AC-吡咯与CB之间吸附能最强(-0.84 eV)的吡咯N基团被确定为CB的关键吸附位点。含有更多吡咯 N 基团的 ACM600 显著提高了化学吸附效果,总吸附量从 8.18 % 增加到 15.12 %。CB 在 ACs 上的吸附机理为物理吸附和弱化学吸附,这归因于π-π堆积作用和氢键的协同作用,其中π-π堆积作用在吸附相互作用中占主导地位。杂原子 N 的引入提高了 AC 的化学反应活性和 π 电子密度分布,与 π 受体形成了更显著的 π-π 堆积相互作用,从而增强了吸附能力。该研究为二恶英减排碳质材料的设计和估算提供了可靠的理论指导和科学依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


