Synthesis and characterisation of Vanadium(III) fluorides α-BaVF5 and BaV(F,OH)5 with an S = 1 spin chain structure
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
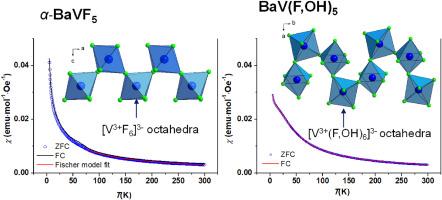
Two new vanadium(III) fluorides, α-BaVF5 and BaV(F,OH)5, were hydrothermally synthesised. The crystal structures of both α-BaVF5 and BaV(F,OH)5 contain chain structures consisting of corner-sharing VIIIF6 or VIII(F,OH)6 octahedra. A monomeric cis-chain structure is found in α-BaVF5, in contrast to the helical-chain structure in BaV(F,OH)5. Although predominantly antiferromagnetic interactions are present in these tilted compounds at high temperatures, the strength of the exchange interaction is greater in α-BaVF5 due to the high Curie−Weiss temperature of θCW = −66.0(1)K. Furthermore, magnetic measurements revealed that α-BaVF5 exhibits a canted antiferromagnetic ground state, while BaV(F,OH)5 displays antiferromagnetic behaviour at low temperatures.
具有 S = 1 自旋链结构的氟化钒(III)α-BaVF5 和 BaV(F,OH)5 的合成与表征
通过水热法合成了两种新的氟化钒(III):α-BaVF5 和 BaV(F,OH)5。α-BaVF5和BaV(F,OH)5的晶体结构都包含由分角VIIIF6或VIII(F,OH)6八面体组成的链结构。虽然这些倾斜化合物在高温下主要存在反铁磁相互作用,但由于 α-BaVF5 的居里-魏斯温度高达 θCW = -66.0(1)K,因此交换相互作用的强度更大。此外,磁性测量结果表明,α-BaVF5 表现出一种倾斜的反铁磁基态,而 BaV(F,OH)5 则在低温下表现出反铁磁性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


