Photocatalytic performance of Ni-Al LDH @Ag2XO4 (X = Cr, Mo, and W) nanocomposites under visible light
IF 5.3
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
The contamination of water sources from dye discharge poses a significant environmental challenge. This study addresses this issue by synthesizing binary composites of Ni-Al LDH@Ag2XO4 (with X=Cr, Mo, and W). The main goal is to increase the separation of charge carriers to boost the efficiency of photocatalysis. The prepared samples were analyzed using FT-IR, FE-SEM, EDS, FE-TEM, XRD, UV–Vis DRS, and XPS techniques. Observations revealed a notable increase in MB degradation through photocatalysis under a 150 W mercury lamp in presence of Ni-Al LDH@Ag2CrO4 compared to individual samples, Ni-Al LDH and Ag2CrO4. At pH= 11, 0.5 g of Ni-Al LDH@Ag2CrO4 shows the highest activity (100 %) for the photodegradation of MB. The absorption edge of Ni-Al LDH@Ag2CrO4 (1.69 eV) has increased compared to that of Ni-Al LDH (2.53 eV), which increases the light absorption capacity. Moreover, the synergistic effect of Ni-Al LDH and Ag2CrO4 increases photocatalytic activity by reducing electron-hole recombination. The proposed Z-Scheme mechanism confirms effective charge separation and increased photocatalytic efficiency.
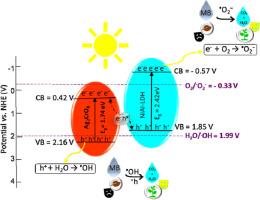
Ni-Al LDH @Ag2XO4(X = Cr、Mo 和 W)纳米复合材料在可见光下的光催化性能
染料排放对水源的污染是一项重大的环境挑战。本研究通过合成 Ni-Al LDH@Ag2XO4 的二元复合材料(X=Cr、Mo 和 W)来解决这一问题。主要目的是增加电荷载流子的分离,从而提高光催化效率。利用傅立叶变换红外光谱、FE-SEM、EDS、FE-TEM、XRD、UV-Vis DRS 和 XPS 技术对制备的样品进行了分析。观察结果表明,与单个样品、Ni-Al LDH 和 Ag2CrO4 相比,在 150 W 汞灯下,Ni-Al LDH@Ag2CrO4 通过光催化降解甲基溴的能力显著提高。在 pH= 11 时,0.5 克 Ni-Al LDH@Ag2CrO4 对甲基溴的光降解活性最高(100%)。与 Ni-Al LDH(2.53 eV)相比,Ni-Al LDH@Ag2CrO4 的吸收边沿(1.69 eV)有所增加,从而提高了光吸收能力。此外,Ni-Al LDH 和 Ag2CrO4 的协同效应通过减少电子-空穴重组提高了光催化活性。提出的 Z-Scheme 机制证实了有效的电荷分离和光催化效率的提高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Research Bulletin
工程技术-材料科学:综合
CiteScore
9.80
自引率
5.60%
发文量
372
审稿时长
42 days
期刊介绍:
Materials Research Bulletin is an international journal reporting high-impact research on processing-structure-property relationships in functional materials and nanomaterials with interesting electronic, magnetic, optical, thermal, mechanical or catalytic properties. Papers purely on thermodynamics or theoretical calculations (e.g., density functional theory) do not fall within the scope of the journal unless they also demonstrate a clear link to physical properties. Topics covered include functional materials (e.g., dielectrics, pyroelectrics, piezoelectrics, ferroelectrics, relaxors, thermoelectrics, etc.); electrochemistry and solid-state ionics (e.g., photovoltaics, batteries, sensors, and fuel cells); nanomaterials, graphene, and nanocomposites; luminescence and photocatalysis; crystal-structure and defect-structure analysis; novel electronics; non-crystalline solids; flexible electronics; protein-material interactions; and polymeric ion-exchange membranes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


