Green tea polyphenol alleviates silica particle-induced lung injury by suppressing IL-17/NF-κB p65 signaling-driven inflammation
IF 6.7
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Background
Silicosis, an interstitial lung disease caused by inhalation of silica particles, poses a significant health concern globally. Green tea polyphenol (TP) stands out as a promising therapeutic candidate, yet its specific protective effects and in-depth mechanisms against silicosis have not been thoroughly investigated.
Purpose
This study aimed to systematically assess the protective potential of TP against silicosis and to elucidate the underlying mechanisms of its action.
Methods
A combination of physiological, transcriptomic, molecular, and computational techniques was employed. HPLC was used to identify the components of TP, and its antioxidant properties were tested with DPPH and ABTS assays. The effects of TP on lung injury were assessed in silicosis mice using histopathology, qRT-PCR, and western blot. Transcriptomic analysis was applied to explore the differentially expressed genes and pathways in response to TP intervention. In vitro studies with mouse alveolar macrophages (MH-S) examined TP's effects on cell viability, proliferation, apoptosis, and inflammation responses. Integrated qRT-PCR, western blot, immunohistochemistry, and molecular docking were performed to confirm the molecular mechanism underlying the protective effects of TP against silicosis.
Results
TP effectively attenuated pulmonary inflammation and fibrosis in silicosis mice, as evidenced by significant reductions in inflammation and fibrotic markers. Moreover, TP's therapeutic benefits were linked to its cytoprotective effects on alveolar macrophages, notably its ability to protect MH-S cells from silica particle-induced apoptosis, inhibition of proliferation, and inflammatory response, underscoring its targeted protective effects at the cellular level. Mechanistically, TP exerted its anti-silicosis activity by targeting key pathways implicated in inflammatory responses, notably through the inhibition of the IL-17/NF-κB p65 signaling cascade. Molecular docking simulations corroborated these findings, demonstrating favorable binding affinities between TP's bioactive components (EGC, ECG, and EGCG) and crucial proteins (IL-17A, IL-17F, p65, TNF-α, IL-6, and IL-1β) involved in the IL-17/NF-κB p65 signaling pathway. This pathway inhibition led to a significant decrease in the production of pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-1β, thus mitigated silicosis.
Conclusion
TP demonstrates efficacy in alleviating silica particle-induced lung injury by suppressing inflammation through the IL-17/NF-κB p65 signaling pathway, underscoring its potential as a valuable natural compound for silicosis management.
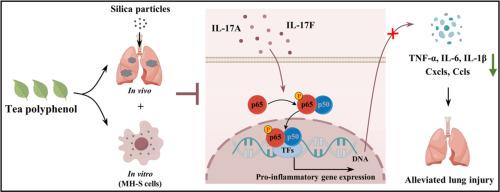
绿茶多酚通过抑制IL-17/NF-κB p65信号驱动的炎症,减轻二氧化硅颗粒诱发的肺损伤。
背景:矽肺病是一种由吸入二氧化硅颗粒引起的间质性肺病,是全球关注的重大健康问题。目的:本研究旨在系统评估绿茶多酚(TP)对矽肺病的保护潜力,并阐明其潜在的作用机制:方法:综合运用生理学、转录组学、分子和计算技术。采用高效液相色谱法鉴定矽肺的成分,并通过 DPPH 和 ABTS 检测法测试其抗氧化特性。使用组织病理学、qRT-PCR 和 Western 印迹技术评估了 TP 对矽肺小鼠肺损伤的影响。转录组分析被用来探索对 TP 干预反应有不同表达的基因和通路。对小鼠肺泡巨噬细胞(MH-S)的体外研究检验了 TP 对细胞活力、增殖、凋亡和炎症反应的影响。此外,还进行了 qRT-PCR、Western 印迹、免疫组织化学和分子对接等综合研究,以确认 TP 对矽肺病具有保护作用的分子机制:结果:TP能有效减轻矽肺小鼠的肺部炎症和纤维化,这体现在炎症和纤维化标志物的显著减少上。此外,TP 的治疗效果还与它对肺泡巨噬细胞的细胞保护作用有关,特别是它能够保护 MH-S 细胞免受二氧化硅颗粒诱导的凋亡、增殖抑制和炎症反应的影响,强调了它在细胞水平上的靶向保护作用。从机理上讲,TP 通过靶向与炎症反应有关的关键通路,特别是通过抑制 IL-17/NF-κB p65 信号级联来发挥其抗硅肺病活性。分子对接模拟证实了这些发现,证明了 TP 的生物活性成分(EGC、ECG 和 EGCG)与参与 IL-17/NF-κB p65 信号通路的关键蛋白(IL-17A、IL-17F、p65、TNF-α、IL-6 和 IL-1β)之间良好的结合亲和力。通过抑制这一途径,TNF-α、IL-6 和 IL-1β 等促炎细胞因子的产生显著减少,从而缓解了矽肺病:TP通过抑制IL-17/NF-κB p65信号通路的炎症反应,在减轻二氧化硅颗粒诱发的肺损伤方面具有疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


