Optimization of continuous spin-freeze-drying: The role of spin-freezing on quality attributes and drying efficiency of a model peptide formulation
IF 4.3
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
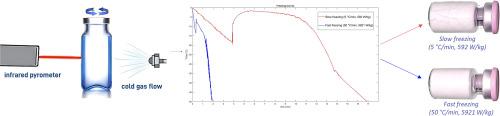
Continuous spin-freeze-drying is an innovative pharmaceutical manufacturing approach offering real-time monitoring and control at the individual vial level, unlike conventional batch lyophilization. A central feature of this technology is spin-freezing, which involves rapidly spinning liquid-filled vials under a precisely controlled cold gas flow, resulting in a thin, uniform frozen product layer. Using a model peptide formulation, we investigated the impact of different cooling and crystallization rates on quality attributes (QA) and primary drying duration. Key QAs included monomer content, peptide assay, moisture content, and pore structure. The monomer content, peptide content, and primary drying duration remained consistent across all spin-freezing conditions. However, scanning electron microscopy (SEM) and Karl Fischer titration revealed that freezing parameters significantly influenced pore structure and residual moisture content. Samples with smaller pores displayed lower residual moisture, as larger surface areas facilitate moisture desorption. Variations in freezing parameters also significantly impacted desorption kinetics during secondary drying. Slower crystallization rates led to more cracks and less shrinkage in the cake structure, while faster rates resulted in more uniform, stable cakes. Although specific to the product under study, these findings highlight the crucial role of spin-freezing in enhancing freeze-drying efficiency and product quality of biopharmaceuticals.
连续旋转冷冻干燥的优化:旋转冷冻对肽制剂模型的质量属性和干燥效率的影响
连续旋转冷冻干燥是一种创新的制药方法,与传统的批量冻干不同,它能在单个小瓶层面进行实时监测和控制。该技术的核心特点是旋转冷冻,即在精确控制的冷气流下快速旋转装满液体的小瓶,从而形成薄而均匀的冷冻产品层。我们使用一个肽配方模型,研究了不同冷却和结晶速率对质量属性(QA)和一次干燥持续时间的影响。关键的质量属性包括单体含量、肽检测、水分含量和孔隙结构。在所有旋转冷冻条件下,单体含量、肽含量和初干持续时间都保持一致。不过,扫描电子显微镜(SEM)和卡尔费休滴定法显示,冷冻参数对孔隙结构和残余水分含量有显著影响。孔隙较小的样品残留水分较低,因为较大的表面积有利于水分的解吸。冷冻参数的变化对二次干燥过程中的解吸动力学也有很大影响。较慢的结晶速度会导致更多的裂缝和更小的饼结构收缩,而较快的结晶速度则会产生更均匀、更稳定的饼。尽管这些研究结果是针对所研究的产品而言的,但它们突出了旋转冷冻在提高生物制药的冷冻干燥效率和产品质量方面的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


