DLGAP3 suppresses malignant behaviors of glioma cells via inhibiting RGS12-mediated MAPK/ERK signaling
IF 2.7
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
Background
Glioma is the most common malignant tumor of the central nervous system, and is characterized by high recurrence, poor prognosis and especially complex pathogenesis. The synaptic plasticity-related protein DLGAP3 is mainly involved in the assembly and function of postsynaptic density complex. It’s widely known that DLGAP3 participating in the occurrence of various neuropsychiatric diseases, but its role in glioma tumorigenesis remains largely unclear.
Methods
We ectopically expressed and knocked down DLGAP3 in glioma cells to perform a series of functional studies in vitro. Meanwhile, western blot analysis, co-immunoprecipitation, enrichment analysis and dual-luciferase reporter system assays were performed to explore the mechanism of DLGAP3 suppressing glioma tumorigenesis and progression.
Results
We found that DLGAP3 was low expressed in gliomas, and decreased DLGAP3 expression was strongly correlated with poor survival of glioma patients. Ectopic expression of DLGAP3 in glioma cell lines dramatically inhibited cell proliferation, invasion and migration. In addition, our data also showed that DLGAP3 can tightly connected with RGS12, and DLGAP3 overexpression significantly increased the expression of RGS12 and inhibited the phosphorylation levels of MEK and ERK. Furthermore, the RGS12 inhibited transcription and translation of BRAF, which further decreased the activity of MAPK/ERK signaling pathway. This suggests that DLGAP3 may act as a tumor suppressor in gliomas and inhibits glioma tumorigenesis by regulating RGS12 and the downstream MAPK/ERK signals axis.
Conclusion
Our data indicates that DLGAP3 is a potential tumor suppressor and valuable prognostic biomarker in gliomas.
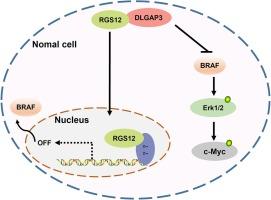
DLGAP3 通过抑制 RGS12 介导的 MAPK/ERK 信号转导抑制胶质瘤细胞的恶性行为。
背景:胶质瘤是中枢神经系统最常见的恶性肿瘤:胶质瘤是中枢神经系统最常见的恶性肿瘤,具有复发率高、预后差、发病机制复杂等特点。突触可塑性相关蛋白 DLGAP3 主要参与突触后密度复合体的组装和功能。众所周知,DLGAP3参与了多种神经精神疾病的发生,但其在胶质瘤肿瘤发生中的作用仍不明确:方法:我们在胶质瘤细胞中异位表达并敲除了 DLGAP3,在体外进行了一系列功能研究。同时,通过Western印迹分析、共免疫沉淀、富集分析和双荧光素酶报告系统实验,探讨DLGAP3抑制胶质瘤肿瘤发生和发展的机制:结果:我们发现DLGAP3在胶质瘤中低表达,且DLGAP3的表达量减少与胶质瘤患者的生存率低密切相关。在胶质瘤细胞系中异位表达 DLGAP3 能显著抑制细胞增殖、侵袭和迁移。此外,我们的数据还显示,DLGAP3与RGS12紧密相连,DLGAP3的过表达能显著增加RGS12的表达,抑制MEK和ERK的磷酸化水平。此外,RGS12还抑制了BRAF的转录和翻译,从而进一步降低了MAPK/ERK信号通路的活性。这表明,DLGAP3可能是胶质瘤的肿瘤抑制因子,通过调节RGS12和下游MAPK/ERK信号轴抑制胶质瘤的肿瘤发生:我们的数据表明,DLGAP3是胶质瘤中潜在的肿瘤抑制因子和有价值的预后生物标志物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


