ECH 1 attenuates atherosclerosis by reducing macrophage infiltration and improving plaque stability through CD36 degradation
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Enoyl coenzyme A hydratase 1 (ECH1) is a secreted protein implicated in numerous metabolic disorders, yet its role in the pathogenesis of atherosclerosis remains unclear. In this study, we found higher serum ECH1 levels in coronary artery disease (CAD) patients and apolipoprotein E (ApoE)−/− mice on a western diet for 12 weeks. In vivo, aorta and aortic sinus histological staining revealed that intraperitoneal injection of recombinant ECH1 reduced aortic lesions, inflammation, and macrophage infiltration in ApoE−/− mice. In vitro, incubating peritoneal macrophages with recombinant ECH1 protein reduced oxidized low-density lipoprotein uptake and increased macrophage migration. Mechanically, we observed that recombinant ECH1 incubation led to a reduction in the protein levels of scavenger receptor cluster of differentiation 36 (CD36) in primary macrophages through the promotion of CD36 protein degradation. Additionally, we found that chloroquine (CQ), a lysosomal inhibitor, mitigated this pro-degradation effect. Taken together, our findings provide unique evidence that ECH1 can attenuate the severity of atherosclerotic plaques, especially improving the stability of plaques, by decreasing macrophage infiltration. ECH1 demonstrates its protective effect by enhancing the lysosome-dependent degradation of CD36, suggesting its potential as a viable target for the prevention and treatment of atherosclerosis.
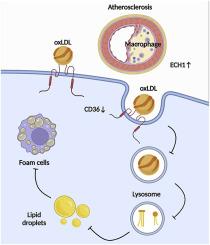
ECH 1 可通过减少巨噬细胞浸润和通过降解 CD36 改善斑块稳定性来减轻动脉粥样硬化。
Enoyl coenzyme A hydratase 1(ECH1)是一种分泌蛋白,与多种代谢紊乱有关,但它在动脉粥样硬化发病机制中的作用仍不清楚。在这项研究中,我们发现冠状动脉疾病(CAD)患者和接受西式饮食 12 周的载脂蛋白 E(ApoE)-/-小鼠血清中的 ECH1 水平较高。体内,主动脉和主动脉窦组织学染色显示,腹腔注射重组 ECH1 可减少载脂蛋白 E-/- 小鼠的主动脉病变、炎症和巨噬细胞浸润。在体外,用重组 ECH1 蛋白培养腹腔巨噬细胞可减少氧化低密度脂蛋白的摄取并增加巨噬细胞的迁移。从机理上讲,我们观察到重组 ECH1 可通过促进 CD36 蛋白降解,降低原代巨噬细胞中清道夫受体分化簇 36(CD36)的蛋白水平。此外,我们还发现溶酶体抑制剂氯喹(CQ)可减轻这种促进降解的作用。综上所述,我们的研究结果提供了独特的证据,证明 ECH1 可以通过减少巨噬细胞的浸润来减轻动脉粥样硬化斑块的严重程度,尤其是改善斑块的稳定性。ECH1 通过增强溶酶体依赖性的 CD36 降解来显示其保护作用,这表明它有可能成为预防和治疗动脉粥样硬化的可行靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


