A NIR-II emissive sonosensitized biotuner for pyroptosis-enhanced sonodynamic therapy of hypoxic tumors
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Pyroptosis is considered as a new way to effectively boost the immune response of tumors and inhibit tumor growth. Effective strategies to induce pyroptosis mainly rely on chemotherapeutic drugs and phototherapy, but their potential biotoxicity and phototoxicity limit their application in biomedicine. Herein, we designed a NIR-II emitting pyroptosis biotuner, Rd-TTPA, which induced pyroptosis under ultrasound irradiation to achieve pyroptosis-enhanced sonodynamic therapy (SDT) and immunogenic cell death (ICD) for tumors. Benefiting from its A-π-D1-D2 structure enhanced donor-acceptor interaction, Rd-TTPA can induce cell pyroptosis under both normoxia (21 % O2) and hypoxia (2 % O2) conditions by rapidly generating superoxide radicals (O2−•) upon ultrasound irradiation. The sonodynamic biotuner of pyroptosis overcomes the longstanding weakness of chemical drug and photosensitizer-based pyroptosis, such as drug resistance and limited penetration depth. In-depth studies demonstrated that Rd-TTPA can selectively target tumor cell mitochondria and possess excellent in vivo NIR-II fluorescence imaging capabilities. Administrating a tumor-bearing mouse model with Rd-TPPA, satisfying antitumor efficacy via pyroptosis-augmented SDT was achieved upon the guidance of NIR-II fluorescence imaging.
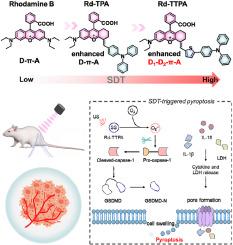
近红外-II发射声敏化生物调谐器,用于缺氧肿瘤的热敏增强声动力疗法。
热休克被认为是有效提高肿瘤免疫反应和抑制肿瘤生长的一种新方法。诱导热休克的有效策略主要依靠化疗药物和光疗,但其潜在的生物毒性和光毒性限制了它们在生物医学中的应用。在此,我们设计了一种发射近红外Ⅱ射线的热休克生物调控剂--Rd-TTPA,它能在超声照射下诱导热休克,从而实现热休克增强声动力疗法(SDT)和肿瘤免疫性细胞死亡(ICD)。Rd-TTPA的A-π-D1-D2结构增强了供体与受体之间的相互作用,在正常缺氧(21% O2)和缺氧(2% O2)条件下,Rd-TTPA都能在超声波照射下通过快速产生超氧自由基(O2--)诱导细胞热解。热释电的声动力生物调谐器克服了长期以来基于化学药物和光敏剂的热释电的弱点,如耐药性和有限的渗透深度。深入研究表明,Rd-TTPA 可选择性地靶向肿瘤细胞线粒体,并具有出色的体内近红外 II 荧光成像能力。在肿瘤小鼠模型中使用Rd-TTPA,在近红外-II荧光成像的指导下,通过热渗透增强的SDT获得了满意的抗肿瘤疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


