Discovery of A Potent Anticancer Agent against Pancreatic Ductal Adenocarcinoma Targeting FAK with DFG-out State and JAK/Aurora Kinases
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
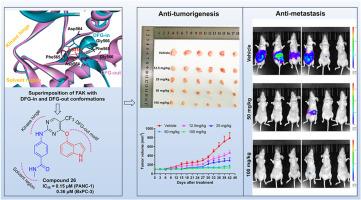
Pancreatic ductal adenocarcinoma (PDAC) is a clinically challenging cancer because of the difficulty in diagnosis and its resistance to chemotherapy. Focal adhesion kinase (FAK) is found overexpressed in PDAC, and targeting FAK has been proved to impede the progress of PDAC. However, most of FAK inhibitors were reported to bind with FAK in a DFG-in conformation, leading to a limited anti-tumor effect in clinical studies. Herein, to develop FAK inhibitors targeting the inactive DFG-out conformation, a series of large aromatic rings were selected to improve the interaction with Phe565 of the DFG motif. Compound 26 was designed to effectively inhibit FAK and the proliferation of PANC-1 cells with IC50 of 50.94 nM and 0.15 μM, respectively. Besides, compound 26 was proved to strongly suppress the proliferation, colony formation, migration, and invasion in FAK-overexpressing PDAC cells. This inhibitor was confirmed to induce the apoptosis and G2/M arrest in PANC-1 cells through the suppression of FAK/PI3K/Akt signal pathway. Meanwhile, compound 26 was found to simultaneously inhibit FAK with DFG-out conformation and JAK3/Aurora B (IC50 of 9.99 nM and 0.49 nM, respectively). In vivo, compound 26 effectively inhibited the tumorigenesis and metastasis of PDAC with desirable biosafety. Overall, these results suggested that compound 26 was a promising candidate for the treatment of PDAC.
发现一种针对胰腺导管腺癌的强效抗癌剂,其靶点是具有 DFG-out 状态的 FAK 和 JAK/Aurora 激酶
胰腺导管腺癌(PDAC)是一种具有临床挑战性的癌症,因为其诊断困难且对化疗耐药。病灶粘附激酶(FAK)在 PDAC 中过度表达,靶向 FAK 已被证实能阻碍 PDAC 的进展。然而,据报道,大多数FAK抑制剂都是以DFG-in构象与FAK结合,导致临床研究中的抗肿瘤效果有限。在此,为了开发针对非活性 DFG-out 构象的 FAK 抑制剂,我们选择了一系列大的芳香环来改善与 DFG 动机的 Phe565 的相互作用。所设计的化合物 26 能有效抑制 FAK 和 PANC-1 细胞的增殖,其 IC50 分别为 50.94 nM 和 0.15 μM。此外,化合物 26 还能强烈抑制过表达 FAK 的 PDAC 细胞的增殖、集落形成、迁移和侵袭。该抑制剂被证实能通过抑制 FAK/PI3K/Akt 信号通路诱导 PANC-1 细胞凋亡和 G2/M 停滞。同时,化合物 26 还能同时抑制具有 DFG-out 构象的 FAK 和 JAK3/Aurora B(IC50 分别为 9.99 nM 和 0.49 nM)。在体内,化合物 26 能有效抑制 PDAC 的肿瘤发生和转移,并具有理想的生物安全性。总之,这些结果表明化合物 26 是一种治疗 PDAC 的有希望的候选化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


