The heterogenous molecular characteristics of biomass-pyrogenic smoke dissolved organic matters (BPS-DOMs) binding with PAHs: Novel insights from combined analysis of FT-ICR MS and fluorescence variation
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
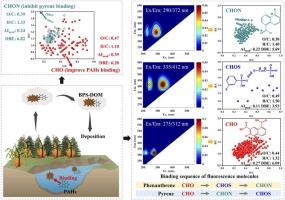
Biomass-pyrogenic smoke dissolved organic matter (BPS-DOM) can co-deposit with polycyclic aromatic hydrocarbons (PAHs), thereby altering their environmental behavior and fate in surface environments. However, the heterogeneous molecular characteristics of BPS-DOM binding with PAHs remain unclear. This study systematically elucidates the binding characteristics of PAHs (phenanthrene and pyrene), with various molecular compositions in BPS-DOM, utilizing FT-ICR MS and fluorescence variation analysis. CHO compounds in BPS-DOM, characterized by high aromaticity and abundant C![]() O bonds, significantly enhance PAHs binding by promoting π-π electron donor-acceptor interactions. In contrast, CHON compounds with higher aliphaticity inhibit pyrene binding by competing for binding sites on BPS-DOM. Furthermore, the binding sequence of different fluorescent molecules follows the order of CHO→CHOS→CHON for phenanthrene and CHO→CHON→CHOS for pyrene. This was primarily due to the larger conjugated aromatic structures of CHO compounds, which provide stronger π-π interaction sites for PAHs binding. The difference in binding sequences between phenanthrene and pyrene is primarily attributed to phenanthrene's reliance on π-π electron donor-acceptor interactions induced by -S
O bonds, significantly enhance PAHs binding by promoting π-π electron donor-acceptor interactions. In contrast, CHON compounds with higher aliphaticity inhibit pyrene binding by competing for binding sites on BPS-DOM. Furthermore, the binding sequence of different fluorescent molecules follows the order of CHO→CHOS→CHON for phenanthrene and CHO→CHON→CHOS for pyrene. This was primarily due to the larger conjugated aromatic structures of CHO compounds, which provide stronger π-π interaction sites for PAHs binding. The difference in binding sequences between phenanthrene and pyrene is primarily attributed to phenanthrene's reliance on π-π electron donor-acceptor interactions induced by -S![]() O and -N = O, while pyrene binding depended on π-π interactions driven by larger conjugated aromatic structures. These results provide an important theoretical foundation for further understanding the molecular-level interactions between BPS-DOM and PAHs.
O and -N = O, while pyrene binding depended on π-π interactions driven by larger conjugated aromatic structures. These results provide an important theoretical foundation for further understanding the molecular-level interactions between BPS-DOM and PAHs.
生物质烟气溶解有机物(BPS-DOMs)与多环芳烃结合的异质分子特征:从 FT-ICR MS 和荧光变化的联合分析中获得新见解
生物质气相烟雾溶解有机物(BPS-DOM)可与多环芳烃(PAHs)共同沉积,从而改变其在地表环境中的环境行为和归宿。然而,BPS-DOM 与多环芳烃结合的异质分子特征仍不清楚。本研究利用 FT-ICR MS 和荧光变化分析,系统地阐明了不同分子组成的 PAHs(菲和芘)与 BPS-DOM 的结合特性。BPS-DOM 中的 CHO 化合物具有高芳香度和丰富的 C=O 键,可通过促进 π-π 电子供体与受体之间的相互作用显著增强 PAHs 的结合力。相反,脂肪族含量较高的 CHON 化合物通过竞争 BPS-DOM 上的结合位点来抑制芘的结合。此外,不同荧光分子的结合顺序依次为 CHO→CHOS→CHON 与菲结合,CHO→CHON→CHOS 与芘结合。这主要是由于 CHO 化合物的共轭芳香结构较大,为多环芳烃的结合提供了更强的π-π相互作用位点。菲和芘在结合顺序上的差异主要归因于菲依赖于 -S=O 和 -N=O 诱导的 π-π 电子供体-受体相互作用,而芘的结合依赖于较大共轭芳香结构驱动的 π-π 相互作用。这些结果为进一步了解 BPS-DOM 与多环芳烃之间的分子级相互作用提供了重要的理论基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


