Isolation and Purification of Protein from Ovis aries Placenta
IF 0.8
4区 化学
Q4 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
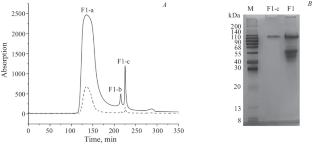
The total protein extract of sheep placenta was fractionated by tandem anion-exchange chromatography over Toyopearl DEAE-650M and gel filtration over Toyopearl HW-55F. The obtained homogeneous protein exhibited antiproliferative activity in HeLa and HCT-116 cell culture (IC50 0.80 ± 0.27 and 1.73 ± 0.03 mg/mL, respectively). The amino-acid composition of the purified protein was determined. Tandem mass spectral sequencing and subsequent annotation using a database identified the purified protein as a calponin homolog.
从羱羊胎盘中分离和纯化蛋白质
羊胎盘总蛋白提取物经串联阴离子交换色谱(Toyopearl DEAE-650M)和凝胶过滤(Toyopearl HW-55F)进行分馏。得到的均质蛋白在 HeLa 和 HCT-116 细胞培养中具有抗增殖活性(IC50 分别为 0.80 ± 0.27 和 1.73 ± 0.03 mg/mL)。测定了纯化蛋白质的氨基酸组成。通过串联质谱测序和随后使用数据库进行注释,确定纯化的蛋白质为钙蛋白同源物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Natural Compounds
化学-有机化学
CiteScore
1.40
自引率
25.00%
发文量
265
审稿时长
7.8 months
期刊介绍:
Chemistry of Natural Compounds publishes reviews and general articles about the structure of different classes of natural compounds, the chemical characteristics of botanical families, genus, and species, to establish the comparative laws and connection between physiological activity and the structure of substances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


