Towards the discovery of unrevealed flufenamic acid cocrystals via structural resemblance for enhanced topical drug delivery
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
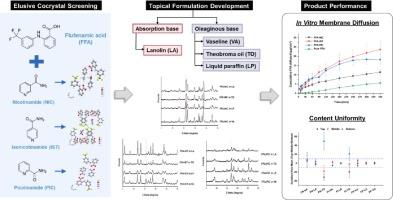
Cocrystallization has emerged as a promising formulation strategy for modulating transdermal drug absorption by enhancing solubility and permeability. However, challenges related to cocrystal dissociation in the semi-solid state need to be addressed to mitigate regulatory concerns before the widespread implementation of topical cocrystal products in clinical practice. This study aimed to develop oil-based topical formulations incorporating cocrystals with distinct thermodynamic stabilities, followed by investigating the roles of different structurally similar coformers and oily vehicles on their physicochemical properties. Three pharmaceutical cocrystals of poorly water-soluble flufenamic acid (FFA) were synthesized with isomeric pyridine carboxamides in a 1:1 stoichiometry via rapid solvent removal. These included the reported flufenamic acid-nicotinamide cocrystal (FFA-NIC), the long-elusive flufenamic acid-isonicotinamide cocrystal (FFA-IST) and flufenamic acid-picolinamide cocrystal (FFA-PIC). The resulting cocrystals, which exhibited different hydrogen bonding patterns, were characterized using powder X-ray diffraction, differential scanning calorimetry, Fourier transform infrared spectroscopy, and structural analysis through single crystal X-ray diffraction. The cocrystals were further formulated in a series of oleaginous and absorption bases, including liquid paraffin, Vaseline, lanolin, and theobroma oil, for topical delivery. The cocrystal dissociation, content uniformity, and in vitro membrane diffusion were assessed. Notably, although all FFA cocrystals exhibited thermodynamic instability in aqueous solution, a significantly reduced propensity for cocrystal dissociation was observed in the ointment bases. Integrated computational analyses of packing efficiency and interaction energy revealed that the thermodynamic stability of cocrystals followed a descending order of FFA-NIC > FFA-PIC > FFA-IST. Compared with raw FFA, FFA-IST and FFA-PIC, which had larger positive ΔVnon-H and ΔEcocryst, achieved superior cumulative diffusion of FFA from Vaseline, with a 4.3-fold (p = 0.0003) and 3.3-fold (p = 0.0029) increase at 6 h in a Franz diffusion cell model, respectively. The diffusion of all FFA cocrystals mainly followed the Higuchi kinetic model and was positively correlated with the intrinsic dissolution rate.
通过结构相似性发现未揭示的氟非那酸共晶体,以增强局部给药效果。
通过提高溶解度和渗透性来调节透皮药物的吸收,结晶已成为一种前景广阔的制剂策略。然而,在半固体状态下与结晶解离有关的挑战需要解决,以减轻监管机构的担忧,然后才能在临床上广泛使用外用结晶产品。本研究旨在开发油基局部制剂,其中含有热力学稳定性不同的共晶体,然后研究不同结构相似的共聚物和油性载体对其理化性质的影响。通过快速去除溶剂,以 1:1 的配比与异构吡啶羧酰胺合成了三种水溶性较差的氟非那酸(FFA)药用共晶体。这些化合物包括已报道的氟非那酸-烟酰胺共晶体(FFA-NIC)、长期未被发现的氟非那酸-异烟酰胺共晶体(FFA-IST)和氟非那酸-吡啶酰胺共晶体(FFA-PIC)。利用粉末 X 射线衍射、差示扫描量热法、傅立叶变换红外光谱以及单晶 X 射线衍射进行了结构分析。这些共晶体被进一步配制在一系列含油基质和吸收基质(包括液体石蜡、凡士林、羊毛脂和可可油)中,用于局部给药。对共晶体的解离、含量均匀性和体外膜扩散进行了评估。值得注意的是,虽然所有的反式脂肪酸茧晶在水溶液中都表现出热力学不稳定性,但在软膏基质中观察到茧晶解离的倾向明显降低。对堆积效率和相互作用能的综合计算分析表明,共晶体的热力学稳定性依次为 FFA-NIC > FFA-PIC > FFA-IST。与未加工的 FFA 相比,FFA-IST 和 FFA-PIC 具有更大的正ΔVnon-H 和 ΔEcocryst,它们实现了更好的 FFA 从凡士林中的累积扩散,在弗兰兹扩散池模型中,6 小时内分别增加了 4.3 倍(p = 0.0003)和 3.3 倍(p = 0.0029)。所有 FFA 共晶体的扩散主要遵循樋口动力学模型,并与固有溶解速率呈正相关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


